3α-Dihydroprogesterone
3α-Dihydroprogesterone (3α-DHP), also known as 3α-hydroxyprogesterone, as well as pregn-4-en-3α-ol-20-one, is an endogenous neurosteroid.[1] It is biosynthesized by 3α-hydroxysteroid dehydrogenase from progesterone.[1] 3α-DHP has been found to act as a positive allosteric modulator of the GABAA receptor and is described as being as active as allopregnanolone in regard to this action.[1] In accordance, it has anxiolytic effects in animals.[2] 3α-DHP has also been found to inhibit the secretion of follicle-stimulating hormone (FSH) from the rat pituitary gland, demonstrating possible antigonadotropic properties.[1] Unlike the case of most other inhibitory neurosteroids, 3α-DHP production is not blocked by 5α-reductase inhibitors like finasteride.[1] Levels of 5α-DHP have been quantified.[3]
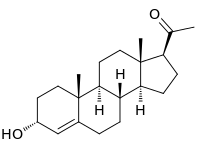 | |
| Names | |
|---|---|
| IUPAC name
3α-Hydroxy-4-pregnen-20-one | |
| Other names
3α-Dihydroprogesterone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H32O2 | |
| Molar mass | 316.485 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
See also
References
- Neurosteroids and Brain Function. Academic Press. 12 December 2001. pp. 484–. ISBN 978-0-08-054423-6.
- Kavaliers, Martin; Wiebe, John P.; Galea, Liisa A.M. (1994). "Reduction of predator odor-induced anxiety in mice by the neurosteroid 3α-hydroxy-4-pregnen-20-one (3αHP)". Brain Research. 645 (1–2): 325–329. doi:10.1016/0006-8993(94)91667-5. ISSN 0006-8993.
- Trabert B, Falk RT, Stanczyk FZ, McGlynn KA, Brinton LA, Xu X (September 2015). "Reproducibility of an assay to measure serum progesterone metabolites that may be related to breast cancer risk using liquid chromatography-tandem mass spectrometry". Horm Mol Biol Clin Investig. 23 (3): 79–84. doi:10.1515/hmbci-2015-0026. PMC 4966666. PMID 26353176.