Etynodiol diacetate
Etynodiol diacetate, or ethynodiol diacetate, sold under the brand names Demulen and Femulen among others, is a progestin medication which is used in birth control pills.[2][3][4] The medication is available only in combination with an estrogen.[5] It is taken by mouth.[6]
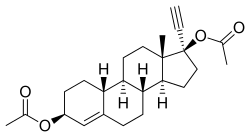 | |
| Clinical data | |
|---|---|
| Trade names | Continuin, Demulen, Femulen, Luteonorm, Luto-Metrodiol, Metrodiol, Ovulen, others |
| Other names | Ethynodiol diacetate; Norethindrol diacetate; 3β-Hydroxynorethisterone 3β,17β-diacetate;[1] 17α-Ethynylestr-4-ene-3β,17β-diyl diacetate; CB-8080; SC-11800 |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.496 |
| Chemical and physical data | |
| Formula | C24H32O4 |
| Molar mass | 384.516 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etynodiol diacetate is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[7][8] It has weak androgenic and estrogenic activity and no other important hormonal activity.[9][10][11] The medication is a prodrug of norethisterone in the body, with etynodiol occurring as an intermediate.[7][8][12]
Etynodiol, a related compound, was discovered in 1954, and etynodiol diacetate was introduced for medical use in 1965.[13][14] The medication remains available today only in the United States, Canada, and a few other countries.[4][5]
Medical uses
Etynodiol diacetate is used in combination with an estrogen such as ethinylestradiol or mestranol in combined oral contraceptives for women.[6]
Side effects
Pharmacology
Etynodiol diacetate is virtually inactive in terms of affinity for the progesterone and androgen receptors and acts as a rapidly converted prodrug of norethisterone, with etynodiol occurring as an intermediate.[7][8][12] Upon oral administration and during first-pass metabolism in the liver, etynodiol diacetate is rapidly converted by esterases into etynodiol,[12] which is followed by oxygenation of the C3 hydroxyl group to produce norethisterone.[8] In addition to its progestogenic activity, etynodiol diacetate has weak androgenic activity,[9][10] and, unlike most progestins but similarly to norethisterone and noretynodrel,[15] also has some estrogenic activity.[10][11]
The pharmacokinetics of etynodiol diacetate have been reviewed.[16]
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
Chemistry
Etynodiol diacetate, also known as 3β-hydroxy-17α-ethynyl-19-nortestosterone 3β,17β-diaceate, 3β-hydroxynorethisterone 3β,17β-diacetate, or 17α-ethynylestr-4-ene-3β,17β-diol 3β,17β-diacetate, is a synthetic estrane steroid and a derivative of testosterone.[1][3][4] It is specifically a derivative of 19-nortestosterone and 17α-ethynyltestosterone, or of norethisterone (17α-ethynyl-19-nortestosterone), in which the C3 ketone group has been dehydrogenated into a C3β hydroxyl group and acetate esters have been attached at the C3β and C17β positions.[3][4] Etynodiol diacetate is the 3β,17β-diacetate ester of etynodiol (17α-ethynylestr-4-ene-3β,17β-diol).[3][4]
Synthesis
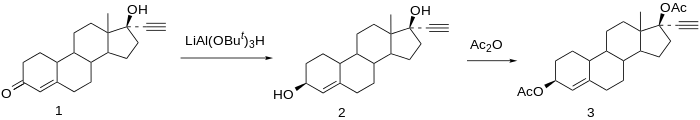
Chemical syntheses of etynodiol diacetate have been published.[16]
Reduction of norethisterone (1) affords the 3,17-diol. The 3β-hydroxy compound is the desired product; since reactions at C3 do not show nearly the stereoselectivity as those at C17 by virtue of the relative lack of stereo-directing proximate substituents, the formation of the desired isomer is engendered by use of a bulky reducing agent, lithium tri-tert-butoxyaluminum hydride. Acetylation of the 3β,17β-diol affords etynodiol diacetate (3).[17]
History
Etynodiol was first synthesized in 1954, via reduction of norethisterone, and etynodiol diacetate was introduced for medical use in 1965.[13][14]
Society and culture
Generic names
Etynodiol diacetate is the generic name of the drug (the INN of its free alcohol form is etynodiol), while ethynodiol diacetate is its USAN, BAN, and JAN.[3][4][5] It is also known by its former developmental code names CB-8080 and SC-11800.[3][4][5]
Brand names
Etynodiol diacetate is or has been marketed under brand names including Conova, Continuin, Demulen, Femulen, Kelnor, Luteonorm, Luto-Metrodiol, Metrodiol, Ovulen, Soluna, Zovia, and others.[3][4][5]
Availability
Etynodiol diacetate remains marketed in only a few countries, including the United States, Canada, Argentina, and Oman.[5]
References
- Schindler, Adolf E; Campagnoli, Carlo; Druckmann, René; Huber, Johannes; Pasqualini, Jorge R; Schweppe, Karl W; Thijssen, Jos H.H (2003). "Classification and pharmacology of progestins". Maturitas. 46: 7–16. doi:10.1016/j.maturitas.2003.09.014. ISSN 0378-5122. PMID 14670641.
- Donna Shoupe; Florence P. Haseltine (6 December 2012). Contraception. Springer Science & Business Media. pp. 21–. ISBN 978-1-4612-2730-4.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 522–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 422. ISBN 978-3-88763-075-1. Retrieved 30 May 2012.
- https://www.drugs.com/international/etynodiol.html
- Robert W. Blum (22 October 2013). Adolescent Health Care: Clinical Issues. Elsevier Science. pp. 216–. ISBN 978-1-4832-7738-7.
- Hammerstein J (1990). "Prodrugs: advantage or disadvantage?". Am. J. Obstet. Gynecol. 163 (6 Pt 2): 2198–203. doi:10.1016/0002-9378(90)90561-K. PMID 2256526.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 146–. ISBN 978-92-832-1291-1.
- Armen H. Tashjian; Ehrin J. Armstrong (21 July 2011). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 523–. ISBN 978-1-4511-1805-6.
- Kenneth L. Becker (24 April 2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. p. 1004. ISBN 978-0-7817-1750-2. Retrieved 30 May 2012.
- Allan H. Goroll; Albert G. Mulley (27 January 2009). Primary Care Medicine: Office Evaluation and Management of the Adult Patient. Lippincott Williams & Wilkins. p. 876. ISBN 978-0-7817-7513-7. Retrieved 30 May 2012.
- Stanczyk FZ (2002). "Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception". Rev Endocr Metab Disord. 3 (3): 211–24. doi:10.1023/A:1020072325818. PMID 12215716.
- Progress in Medicinal Chemistry. Butterworth-Heinemann. 21 September 2011. pp. 180–. ISBN 978-0-08-086256-9.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1516–. ISBN 978-0-8155-1856-3.
- Benno Clemens Runnebaum; Thomas Rabe; Ludwig Kiesel (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 36–. ISBN 978-3-642-73790-9.
- Die Gestagene. Springer-Verlag. 27 November 2013. pp. 14–15, 286. ISBN 978-3-642-99941-3.
- Klimstra, P.; Colton, F. (1967). "The synthesis of 3β-hydroxyestr-4-en-17-one and 3β-hydroxiandrost-4-en-17-one". Steroids. 10 (4): 411–424. doi:10.1016/0039-128X(67)90119-5.
- Sondheimer, F.; Klibansky, Y. (1959). "Synthesis of 3β-hydroxy analogues of steroidal hormones, a biologically active class of compounds". Tetrahedron. 5: 15–26. doi:10.1016/0040-4020(59)80066-1.