Coronavirus disease 2019
Coronavirus disease 2019 (COVID‑19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[9] It was first identified in December 2019 in Wuhan, Hubei, China, and has resulted in an ongoing pandemic.[10][11] As of 15 August 2020, more than 21.2 million cases have been reported across 188 countries and territories, resulting in more than 767,000 deaths. More than 13.2 million people have recovered.[8]
| Coronavirus disease 2019 (COVID-19) | |
|---|---|
| Other names | |
 | |
False-color transmission electron microscope image of Coronavirus | |
| Pronunciation |
|
| Specialty | Infectious disease |
| Symptoms | Fever, cough, fatigue, shortness of breath, loss of smell; sometimes no symptoms at all[5][6] |
| Complications | Pneumonia, viral sepsis, acute respiratory distress syndrome, kidney failure, cytokine release syndrome |
| Usual onset | 2–14 days (typically 5) from infection |
| Causes | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
| Diagnostic method | rRT-PCR testing, CT scan |
| Prevention | Hand washing, face coverings, quarantine, social distancing[7] |
| Treatment | Symptomatic and supportive |
| Frequency | 21,280,608[8] confirmed cases |
| Deaths | 767,422 (3.6% of confirmed cases)[8] |
| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|
International response |
|
Medical response |
|
|
|
Common symptoms include fever, cough, fatigue, shortness of breath, and loss of smell and taste.[12][5][6][13] While most people have mild symptoms, some people develop acute respiratory distress syndrome (ARDS) possibly precipitated by cytokine storm,[14] multi-organ failure, septic shock, and blood clots.[15][16][17] The time from exposure to onset of symptoms is typically around five days, but may range from two to fourteen days.[5][18]
The virus is spread primarily via small droplets produced by coughing,[lower-alpha 1] sneezing, and talking.[6][20][21] The droplets usually fall to the ground or onto surfaces rather than travelling through air over long distances.[6][22] However, those standing in close proximity may inhale these droplets and become infected.[lower-alpha 2] People may also become infected by touching a contaminated surface and then touching their face.[6][20] The transmission may also occur through smaller droplets that are able to stay suspended in the air for longer periods of time in enclosed spaces, as typical for airborne diseases.[23] It is most contagious during the first three days after the onset of symptoms, although spread is possible before symptoms appear, and from people who do not show symptoms.[6][20] The standard method of diagnosis is by real-time reverse transcription polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab.[24] Chest CT imaging may also be helpful for diagnosis in individuals where there is a high suspicion of infection based on symptoms and risk factors; however, guidelines do not recommend using CT imaging for routine screening.[25][26]
Recommended measures to prevent infection include frequent hand washing, maintaining physical distance from others (especially from those with symptoms), quarantine (especially for those with symptoms), covering coughs, and keeping unwashed hands away from the face.[7][27][28] The use of cloth face coverings such as a scarf or a bandana has been recommended by health officials in public settings to minimise the risk of transmissions, with some authorities requiring their use.[29][30] Health officials also stated that medical-grade face masks, such as N95 masks, should be used only by healthcare workers, first responders, and those who directly care for infected individuals.[31][32]
There are no proven vaccines nor specific antiviral treatments for COVID-19.[6] Management involves the treatment of symptoms, supportive care, isolation, and experimental measures.[33] The World Health Organization (WHO) declared the COVID‑19 outbreak a public health emergency of international concern (PHEIC)[34][35] on 30 January 2020 and a pandemic on 11 March 2020.[11] Local transmission of the disease has occurred in most countries across all six WHO regions.[36]
Signs and symptoms
| Symptom | Range |
|---|---|
| Fever | 83–99% |
| Cough | 59–82% |
| Loss of appetite | 40–84% |
| Fatigue | 44–70% |
| Shortness of breath | 31–40% |
| Coughing up sputum | 28–33% |
| Muscle aches and pains | 11–35% |

Symptoms of COVID-19 are variable, but usually include fever and a cough.[12][5] People with the same infection may have different symptoms, and their symptoms may change over time. For example, one person may have a high fever, a cough, and fatigue, and another person may have a low fever at the start of the disease and develop difficulty breathing a week later. All of the symptoms of COVID-19 are non-specific, which means that they are also seen in some other diseases.
Fever is the most common symptom of COVID‑19.[12] The fever may be high or low. Most people with COVID-19 develop a fever at some point.[12] Most people with COVID-19 also have a cough, which could be either dry or a productive cough.[12]
Other typical symptoms include fatigue, shortness of breath and muscle and joint pains.[12][5] Some symptoms, such as difficulty breathing, are more common in patients who need hospital care.[5] Shortness of breath tends to develop later in the illness.
About 40% of people temporarily lose their sense of smell (called anosmia), experience changes in how food tastes (dysgeusia), or have other disturbances to their normal abilities to smell or taste.[5][38] This symptom, if it is present at all, often appears early in the illness.[38] A disturbance in smell or taste is more commonly found in younger people, and perhaps because of this, it is associated with a lower risk of medical complications.[38] Although most people with COVID-19 do not experience these symptoms, it is an unusual symptom for other respiratory diseases, so it is used for symptom-based screening.[38]
Other symptoms are less common among people with COVID-19. Some people experience gastrointestinal symptoms such as loss of appetite, diarrhea, or nausea.[5][39] Some people have a sore throat, headache, vertigo, or other symptoms.[12][5]
As is common with infections, there is a delay between the moment a person first becomes infected and the appearance of the first symptoms. This delay is called the incubation period. The median incubation period for COVID‑19 is four to five days.[40] Most symptomatic people experience symptoms within two to seven days after exposure, and almost all symptomatic people will experience one or more symptoms before day twelve.[40][41]
Some people are infected with the virus but do not develop noticeable symptoms at any point in time.[42] These asymptomatic carriers tend not to get tested, and they can spread the disease.[43][44][42] Other infected people will develop symptoms later (called pre-symptomatic) or have very mild symptoms (called paucisymptomatic), and can also spread the virus.
Cause
COVID-19 is caused by infection with the Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus strain.
Transmission
COVID‑19 is a new disease, and many of the details of its spread are still under investigation.[6][20][21] It spreads easily between people—more easily than influenza but not as easily as measles.[20] People are most infectious when they show symptoms (even mild or non-specific symptoms), but may be infectious for up to two days before symptoms appear (pre-symptomatic transmission).[21] They remain infectious for an estimated seven to twelve days in moderate cases and an average of two weeks in severe cases.[21] People can also transmit the virus without showing any symptom (asymptomatic transmission), but it is unclear how often this happens.[6][20][21] A June 2020 review found that 40–45% of infected people are asymptomatic.[45]
COVID‑19 spreads primarily when people are in close contact and one person inhales small droplets produced by an infected person (symptomatic or not) coughing, sneezing, talking, or singing.[21][46] The WHO recommends 1 metre (3 ft) of social distance;[6] the US Centers for Disease Control and Prevention (CDC) recommends 2 metres (6 ft).[20]
Transmission may also occur through aerosols, smaller droplets that are able to stay suspended in the air for longer periods of time.[23] Experimental results show the virus can survive in aerosol for up to three hours.[47] Some outbreaks have also been reported in crowded and inadequately ventilated indoor locations where infected persons spend long periods of time (such as restaurants and nightclubs).[48] Aerosol transmission in such locations has not been ruled out.[23] Some medical procedures performed on COVID‑19 patients in health facilities can generate those smaller droplets,[49] and result in the virus being transmitted more easily than normal.[6][21]
Less commonly, when the contaminated droplets fall to floors or surfaces they can remain infectious if people touch contaminated surfaces and then their eyes, nose or mouth with unwashed hands.[6] On surfaces the amount of viable active virus decreases over time until it can no longer cause infection,[21] and surfaces are thought not to be the main way the virus spreads.[20] The level of contamination required to transmit infection via surfaces is unknown, but the virus can be detected for up to four hours on copper, up to one day on cardboard, and up to three days on plastic (polypropylene) and stainless steel (AISI 304).[21][50][51] Surfaces are easily decontaminated with household disinfectants which destroy the virus outside the human body or on the hands.[6] Disinfectants or bleach are not a treatment for COVID‑19, and cause health problems when not used properly, such as when used inside the human body.[52]
Sputum and saliva carry large amounts of virus.[6][20][21][53] Although COVID‑19 is not a sexually transmitted infection, direct contact such as kissing, intimate contact, and fecal–oral routes are suspected to transmit the virus.[54][55] The virus may occur in breast milk, but whether it is transmittable to the baby is unknown.[56][57]
Estimates of the number of people infected by one person with COVID‑19, the R0, have varied. The WHO's initial estimates of R0 were 1.4–2.5 (average 1.95); however, a review in early April 2020 found the basic R0 (without control measures) to be higher at 3.28 and the median R0 to be 2.79.[58]
Virology
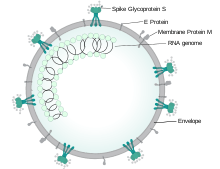
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel severe acute respiratory syndrome coronavirus. It was first isolated from three people with pneumonia connected to the cluster of acute respiratory illness cases in Wuhan.[59] All features of the novel SARS-CoV-2 virus occur in related coronaviruses in nature.[60]
Outside the human body, the virus is destroyed by household soap, which bursts its protective bubble.[25]
SARS-CoV-2 is closely related to the original SARS-CoV.[61] It is thought to have an animal (zoonotic) origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus, in subgenus Sarbecovirus (lineage B) together with two bat-derived strains. It is 96% identical at the whole genome level to other bat coronavirus samples (BatCov RaTG13).[62]
Pathophysiology
COVID‑19 can affect the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs).[63] The lungs are the organs most affected by COVID‑19 because the virus accesses host cells via the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant in type II alveolar cells of the lungs.[64] The virus uses a special surface glycoprotein called a "spike" (peplomer) to connect to ACE2 and enter the host cell.[65] The density of ACE2 in each tissue correlates with the severity of the disease in that tissue and some have suggested decreasing ACE2 activity might be protective,[66][67] though another view is that increasing ACE2 using angiotensin II receptor blocker medications could be protective.[68] As the alveolar disease progresses, respiratory failure might develop and death may follow.[67]
SARS-CoV-2 may also cause respiratory failure through affecting the brainstem as other coronaviruses have been found to invade the central nervous system (CNS). While virus has been detected in cerebrospinal fluid of autopsies, the exact mechanism by which it invades the CNS remains unclear and may first involve invasion of peripheral nerves given the low levels of ACE2 in the brain.[69][70]
The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelium[71] as well as endothelial cells and enterocytes of the small intestine.[72]
The virus can cause acute myocardial injury and chronic damage to the cardiovascular system.[73] An acute cardiac injury was found in 12% of infected people admitted to the hospital in Wuhan, China,[74] and is more frequent in severe disease.[75] Rates of cardiovascular symptoms are high, owing to the systemic inflammatory response and immune system disorders during disease progression, but acute myocardial injuries may also be related to ACE2 receptors in the heart.[73] ACE2 receptors are highly expressed in the heart and are involved in heart function.[73][76] A high incidence of thrombosis (31%) and venous thromboembolism (25%) have been found in ICU patients with COVID‑19 infections, and may be related to poor prognosis.[77][78] Blood vessel dysfunction and clot formation (as suggested by high D-dimer levels) are thought to play a significant role in mortality, incidences of clots leading to pulmonary embolisms, and ischaemic events within the brain have been noted as complications leading to death in patients infected with SARS-CoV-2. Infection appears to set off a chain of vasoconstrictive responses within the body, constriction of blood vessels within the pulmonary circulation has also been posited as a mechanism in which oxygenation decreases alongside the presentation of viral pneumonia.[79]
Another common cause of death is complications related to the kidneys.[79] Early reports show that up to 30% of hospitalized patients both in China and in New York have experienced some injury to their kidneys, including some persons with no previous kidney problems.[80]
Autopsies of people who died of COVID‑19 have found diffuse alveolar damage (DAD), and lymphocyte-containing inflammatory infiltrates within the lung.[81]
Immunopathology
Although SARS-CoV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, patients with severe COVID‑19 have symptoms of systemic hyperinflammation. Clinical laboratory findings of elevated IL-2, IL-7, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1-α (MIP-1α), and tumour necrosis factor-α (TNF-α) indicative of cytokine release syndrome (CRS) suggest an underlying immunopathology.[74]
Additionally, people with COVID‑19 and acute respiratory distress syndrome (ARDS) have classical serum biomarkers of CRS, including elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.[82]
Systemic inflammation results in vasodilation, allowing inflammatory lymphocytic and monocytic infiltration of the lung and the heart. In particular, pathogenic GM-CSF-secreting T-cells were shown to correlate with the recruitment of inflammatory IL-6-secreting monocytes and severe lung pathology in COVID‑19 patients. Lymphocytic infiltrates have also been reported at autopsy.[81]
Diagnosis
.jpg)
The WHO has published several testing protocols for the disease.[84] The standard method of testing is real-time reverse transcription polymerase chain reaction (rRT-PCR).[85] The test is typically done on respiratory samples obtained by a nasopharyngeal swab; however, a nasal swab or sputum sample may also be used.[24][86] Results are generally available within a few hours to two days.[87][88] Blood tests can be used, but these require two blood samples taken two weeks apart, and the results have little immediate value.[89] Chinese scientists were able to isolate a strain of the coronavirus and publish the genetic sequence so laboratories across the world could independently develop polymerase chain reaction (PCR) tests to detect infection by the virus.[10][90][91] As of 4 April 2020, antibody tests (which may detect active infections and whether a person had been infected in the past) were in development, but not yet widely used.[92][93][94] Antibody tests may be most accurate 2–3 weeks after a person's symptoms start.[95] The Chinese experience with testing has shown the accuracy is only 60 to 70%.[96] The US Food and Drug Administration (FDA) approved the first point-of-care test on 21 March 2020 for use at the end of that month.[97] The absence or presence of COVID‑19 signs and symptoms alone is not reliable enough for an accurate diagnosis.[98]
Diagnostic guidelines released by Zhongnan Hospital of Wuhan University suggested methods for detecting infections based upon clinical features and epidemiological risk. These involved identifying people who had at least two of the following symptoms in addition to a history of travel to Wuhan or contact with other infected people: fever, imaging features of pneumonia, normal or reduced white blood cell count, or reduced lymphocyte count.[99]
A study asked hospitalised COVID‑19 patients to cough into a sterile container, thus producing a saliva sample, and detected the virus in eleven of twelve patients using RT-PCR. This technique has the potential of being quicker than a swab and involving less risk to health care workers (collection at home or in the car).[53]
Along with laboratory testing, chest CT scans may be helpful to diagnose COVID‑19 in individuals with a high clinical suspicion of infection but are not recommended for routine screening.[25][26] Bilateral multilobar ground-glass opacities with a peripheral, asymmetric, and posterior distribution are common in early infection.[25] Subpleural dominance, crazy paving (lobular septal thickening with variable alveolar filling), and consolidation may appear as the disease progresses.[25][100]
In late 2019, the WHO assigned emergency ICD-10 disease codes U07.1 for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID‑19 without lab-confirmed SARS-CoV-2 infection.[101]
 CT scan of rapid progression stage of COVID-19.
CT scan of rapid progression stage of COVID-19. Chest X-ray showing COVID-19 pneumonia.
Chest X-ray showing COVID-19 pneumonia.
Pathology
Few pieces of data are available about microscopic lesions and the pathophysiology of COVID‑19.[102][103] The main pathological findings at autopsy are:
- Macroscopy: pleurisy, pericarditis, lung consolidation and pulmonary oedema
- Four types of severity of viral pneumonia can be observed:
- minor pneumonia: minor serous exudation, minor fibrin exudation
- mild pneumonia: pulmonary oedema, pneumocyte hyperplasia, large atypical pneumocytes, interstitial inflammation with lymphocytic infiltration and multinucleated giant cell formation
- severe pneumonia: diffuse alveolar damage (DAD) with diffuse alveolar exudates. DAD is the cause of acute respiratory distress syndrome (ARDS) and severe hypoxemia.
- healing pneumonia: organisation of exudates in alveolar cavities and pulmonary interstitial fibrosis
- plasmocytosis in BAL[104]
- Blood: disseminated intravascular coagulation (DIC);[105] leukoerythroblastic reaction[106]
- Liver: microvesicular steatosis
Prevention

.gif)
A COVID-19 vaccine is not expected until 2021 at the earliest.[113] The US National Institutes of Health guidelines do not recommend any medication for prevention of COVID‑19, before or after exposure to the SARS-CoV-2 virus, outside the setting of a clinical trial.[114][115] Without a vaccine, other prophylactic measures, or effective treatments, a key part of managing COVID‑19 is trying to decrease and delay the epidemic peak, known as "flattening the curve".[109] This is done by slowing the infection rate to decrease the risk of health services being overwhelmed, allowing for better treatment of current cases, and delaying additional cases until effective treatments or a vaccine become available.[109][112]
Preventive measures to reduce the chances of infection include staying at home, wearing a mask in public, avoiding crowded places, keeping distance from others, washing hands with soap and water often and for at least 20 seconds, practising good respiratory hygiene, and avoiding touching the eyes, nose, or mouth with unwashed hands.[116][117][118][119]
The US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommend individuals wear non-medical face coverings in public settings where there is an increased risk of transmission and where social distancing measures are difficult to maintain.[120][29][121] This recommendation is meant to reduce the spread of the disease by asymptomatic and pre-symtomatic individuals and is complementary to established preventive measures such as social distancing.[29][122] Face coverings limit the volume and travel distance of expiratory droplets dispersed when talking, breathing, and coughing.[29][122] Many countries and local jurisdictions encourage or mandate the use of face masks or cloth face coverings by members of the public to limit the spread of the virus.[123][124][125][126]
Masks are also strongly recommended for those who may have been infected and those taking care of someone who may have the disease.[127] When not wearing a mask, the CDC recommends covering the mouth and nose with a tissue when coughing or sneezing and recommends using the inside of the elbow if no tissue is available.[117] Proper hand hygiene after any cough or sneeze is encouraged.[117]
Social distancing strategies aim to reduce contact of infected persons with large groups by closing schools and workplaces, restricting travel, and cancelling large public gatherings.[128] Distancing guidelines also include that people stay at least 6 feet (1.8 m) apart.[129] After the implementation of social distancing and stay-at-home orders, many regions have been able to sustain an effective transmission rate ("Rt") of less than one, meaning the disease is in remission in those areas.[130]
The CDC also recommends that individuals wash hands often with soap and water for at least 20 seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose, coughing or sneezing. The CDC further recommends using an alcohol-based hand sanitiser with at least 60% alcohol, but only when soap and water are not readily available.[117] For areas where commercial hand sanitisers are not readily available, the WHO provides two formulations for local production. In these formulations, the antimicrobial activity arises from ethanol or isopropanol. Hydrogen peroxide is used to help eliminate bacterial spores in the alcohol; it is "not an active substance for hand antisepsis". Glycerol is added as a humectant.[131]
Those diagnosed with COVID‑19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, wear a face mask before entering the healthcare provider's office and when in any room or vehicle with another person, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.[31][132]
Sanitizing of frequently touched surfaces is also recommended or required by regulation for businesses and public facilities; the United States Environmental Protection Agency maintains a list of products expected to be effective.[133]
On 7 July 2020, the WHO said in a press conference that it will issue new guidelines about airborne transmission in settings with close contact and poor ventilation.[134]
For health care professionals who may come into contact with COVID‑19 positive bodily fluids, using personal protective coverings on exposed body parts improves protection from the virus.[135] Breathable personal protective equipment improves user-satisfaction and may offer a similar level of protection from the virus.[135] In addition, adding tabs and other modifications to the protective equipment may reduce the risk of contamination during donning and doffing (putting on and taking off the equipment).[135] Implementing an evidence-based donning and doffing protocol such as a one-step glove and gown removal technique, giving oral instructions while donning and doffing, double gloving, and the use of glove disinfection may also improve protection for health care professionals.[135]
Management
People are managed with supportive care, which may include fluid therapy, oxygen support, and supporting other affected vital organs.[136][137][138] The CDC recommends those who suspect they carry the virus wear a simple face mask.[31] Extracorporeal membrane oxygenation (ECMO) has been used to address the issue of respiratory failure, but its benefits are still under consideration.[139] Personal hygiene and a healthy lifestyle and diet have been recommended to improve immunity.[140] Supportive treatments may be useful in those with mild symptoms at the early stage of infection.[141]
The WHO, the Chinese National Health Commission, and the United States' National Institutes of Health have published recommendations for taking care of people who are hospitalised with COVID‑19.[114][142][143] Intensivists and pulmonologists in the US have compiled treatment recommendations from various agencies into a free resource, the IBCC.[144][145]
Prognosis
The severity of COVID‑19 varies. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the common cold. Mild cases typically recover within two weeks, while those with severe or critical diseases may take three to six weeks to recover. Among those who have died, the time from symptom onset to death has ranged from two to eight weeks.[62]
Children make up a small proportion of reported cases, with about 1% of cases being under 10 years and 4% aged 10–19 years.[21] They are likely to have milder symptoms and a lower chance of severe disease than adults. In those younger than 50 years the risk of death is less than 0.5%, while in those older than 70 it is more than 8%.[152][153][154] Pregnant women may be at higher risk of severe COVID‑19 infection based on data from other similar viruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), but data for COVID‑19 is lacking.[155][156] According to scientific reviews smokers are more likely to require intensive care or die compared to non-smokers,[157][158] air pollution is similarly associated with risk factors,[158] and obesity contributes to an increased health risk of COVID‑19.[158][159][160]
A European multinational study of hospitalized children published in The Lancet on 25 June 2020 found that about 8% of children admitted to a hospital needed intensive care. Four of those 582 children (0.7%) died, but the actual mortality rate could be "substantially lower" since milder cases that did not seek medical help were not included in the study.[161]
| Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90+ |
| Argentina as of 7 May[162] | 0.0 | 0.0 | 0.1 | 0.4 | 1.3 | 3.6 | 12.9 | 18.8 | 28.4 | |
| Australia as of 4 June[163] | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 1.1 | 4.1 | 18.1 | 40.8 |
| Canada as of 3 June[164] | 0.0 | 0.1 | 0.7 | 11.2 | 30.7 | |||||
| Alberta as of 3 June[165] | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.2 | 1.9 | 11.9 | 30.8 | |
| Br. Columbia as of 2 June[166] | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.8 | 4.6 | 12.3 | 33.8 | 33.6 |
| Ontario as of 3 June[167] | 0.0 | 0.0 | 0.1 | 0.2 | 0.5 | 1.5 | 5.6 | 17.7 | 26.0 | 33.3 |
| Quebec as of 2 June[168] | 0.0 | 0.1 | 0.1 | 0.2 | 1.1 | 6.1 | 21.4 | 30.4 | 36.1 | |
| Chile as of 31 May[169][170] | 0.1 | 0.3 | 0.7 | 2.3 | 7.7 | 15.6 | ||||
| China as of 11 February[171] | 0.0 | 0.2 | 0.2 | 0.2 | 0.4 | 1.3 | 3.6 | 8.0 | 14.8 | |
| Colombia as of 3 June[172] | 0.3 | 0.0 | 0.2 | 0.5 | 1.6 | 3.4 | 9.4 | 18.1 | 25.6 | 35.1 |
| Denmark as of 4 June[173] | 0.2 | 4.1 | 16.5 | 28.1 | 48.2 | |||||
| Finland as of 4 June[174] | 0.0 | 0.0 | <0.4 | <0.4 | <0.5 | 0.8 | 3.8 | 18.1 | 42.3 | |
| Germany as of 5 June[175] | 0.0 | 0.0 | 0.1 | 1.9 | 19.7 | 31.0 | ||||
| Bavaria as of 5 June[176] | 0.0 | 0.0 | 0.1 | 0.1 | 0.2 | 0.9 | 5.4 | 15.8 | 28.0 | 35.8 |
| Israel as of 3 May[177] | 0.0 | 0.0 | 0.0 | 0.9 | 0.9 | 3.1 | 9.7 | 22.9 | 30.8 | 31.3 |
| Italy as of 3 June[178] | 0.3 | 0.0 | 0.1 | 0.3 | 0.9 | 2.7 | 10.6 | 25.9 | 32.4 | 29.9 |
| Japan as of 7 May[179] | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.6 | 2.5 | 6.8 | 14.8 | |
| Mexico as of 3 June[180] | 3.3 | 0.6 | 1.2 | 2.9 | 7.5 | 15.0 | 25.3 | 33.7 | 40.3 | 40.6 |
| Netherlands as of 3 June[181] | 0.0 | 0.2 | 0.1 | 0.3 | 0.5 | 1.7 | 8.1 | 25.6 | 33.3 | 34.5 |
| Norway as of 4 June[182] | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 2.2 | 9.0 | 22.7 | 57.0 |
| Philippines as of 4 June[183] | 1.6 | 0.9 | 0.5 | 0.8 | 2.4 | 5.5 | 13.2 | 20.9 | 31.5 | |
| Portugal as of 3 June[184] | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 1.3 | 3.6 | 10.5 | 21.2 | |
| South Africa as of 28 May[185] | 0.3 | 0.1 | 0.1 | 0.4 | 1.1 | 3.8 | 9.2 | 15.0 | 12.3 | |
| South Korea as of 17 July[186] | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.6 | 2.3 | 9.5 | 25.2 | |
| Spain as of 29 May[187] | 0.3 | 0.2 | 0.2 | 0.3 | 0.6 | 1.4 | 5.0 | 14.3 | 20.8 | 21.7 |
| Sweden as of 5 June[188] | 0.5 | 0.0 | 0.2 | 0.2 | 0.6 | 1.7 | 6.6 | 23.4 | 35.6 | 40.3 |
| Switzerland as of 4 June[189] | 0.6 | 0.0 | 0.0 | 0.1 | 0.1 | 0.6 | 3.4 | 11.6 | 28.2 | |
| United States | ||||||||||
| Colorado as of 3 June[190] | 0.2 | 0.2 | 0.2 | 0.2 | 0.8 | 1.9 | 6.2 | 18.5 | 39.0 | |
| Connecticut as of 3 June[191] | 0.2 | 0.1 | 0.1 | 0.3 | 0.7 | 1.8 | 7.0 | 18.0 | 31.2 | |
| Georgia as of 3 June[192] | 0.0 | 0.1 | 0.5 | 0.9 | 2.0 | 6.1 | 13.2 | 22.0 | ||
| Idaho as of 3 June[193] | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 3.1 | 8.9 | 31.4 | |
| Indiana as of 3 June[194] | 0.1 | 0.1 | 0.2 | 0.6 | 1.8 | 7.3 | 17.1 | 30.2 | ||
| Kentucky as of 20 May[195] | 0.0 | 0.0 | 0.0 | 0.2 | 0.5 | 1.9 | 5.9 | 14.2 | 29.1 | |
| Maryland as of 20 May[196] | 0.0 | 0.1 | 0.2 | 0.3 | 0.7 | 1.9 | 6.1 | 14.6 | 28.8 | |
| Massachusetts as of 20 May[197] | 0.0 | 0.0 | 0.1 | 0.1 | 0.4 | 1.5 | 5.2 | 16.8 | 28.9 | |
| Minnesota as of 13 May[198] | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 1.6 | 5.4 | 26.9 | ||
| Mississippi as of 19 May[199] | 0.0 | 0.1 | 0.5 | 0.9 | 2.1 | 8.1 | 16.1 | 19.4 | 27.2 | |
| Missouri as of 19 May[200] | 0.0 | 0.0 | 0.1 | 0.2 | 0.8 | 2.2 | 6.3 | 14.3 | 22.5 | |
| Nevada as of 20 May[201] | 0.0 | 0.3 | 0.3 | 0.4 | 1.7 | 2.6 | 7.7 | 22.3 | ||
| N. Hampshire as of 12 May[202] | 0.0 | 0.0 | 0.4 | 0.0 | 1.2 | 0.0 | 2.2 | 12.0 | 21.2 | |
| Oregon as of 12 May[203] | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.8 | 5.6 | 12.1 | 28.9 | |
| Texas as of 20 May[204] | 0.0 | 0.5 | 0.4 | 0.3 | 0.8 | 2.1 | 5.5 | 10.1 | 30.6 | |
| Virginia as of 19 May[205] | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 | 1.0 | 4.4 | 12.9 | 24.9 | |
| Washington as of 10 May[206] | 0.0 | 0.2 | 1.3 | 9.8 | 31.2 | |||||
| Wisconsin as of 20 May[207] | 0.0 | 0.0 | 0.2 | 0.2 | 0.6 | 2.0 | 5.0 | 14.7 | 19.9 | 30.4 |
Comorbidities
Most of those who die of COVID‑19 have pre-existing (underlying) conditions, including hypertension, diabetes mellitus, and cardiovascular disease.[208] The Istituto Superiore di Sanità reported that out of 8.8% of deaths where medical charts were available, 97% of people had at least one comorbidity with the average person having 2.7 diseases.[209] According to the same report, the median time between the onset of symptoms and death was ten days, with five being spent hospitalised. However, people transferred to an ICU had a median time of seven days between hospitalisation and death.[209] In a study of early cases, the median time from exhibiting initial symptoms to death was 14 days, with a full range of six to 41 days.[210] In a study by the National Health Commission (NHC) of China, men had a death rate of 2.8% while women had a death rate of 1.7%.[211] In 11.8% of the deaths reported by the National Health Commission of China, heart damage was noted by elevated levels of troponin or cardiac arrest.[212] According to March data from the United States, 89% of those hospitalised had preexisting conditions.[213]
Most critical respiratory comorbidities according to the CDC, are: moderate or severe asthma, pre-existing COPD, pulmonary fibrosis, cystic fibrosis.[214] Current evidence stemming from meta-analysis of several smaller research papers also suggests that smoking can be associated with worse patient outcomes.[215][216] When someone with existing respiratory problems is infected with COVID‑19, they might be at greater risk for severe symptoms.[217] COVID‑19 also poses a greater risk to people who misuse opioids and methamphetamines, insofar as their drug use may have caused lung damage.[218]
Complications and long-term effects
Complications may include pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, septic shock, and death.[10][15][219][220] Cardiovascular complications may include heart failure, arrhythmias, heart inflammation, and blood clots.[221][222][223]
Approximately 20–30% of people who present with COVID‑19 have elevated liver enzymes reflecting liver injury.[224][115]
Neurologic manifestations include seizure, stroke, encephalitis, and Guillain–Barré syndrome (which includes loss of motor functions).[225] Following the infection, children may develop paediatric multisystem inflammatory syndrome, which has symptoms similar to Kawasaki disease, which can be fatal.[226][227]
Concerns have been raised about long-term sequelae of the disease. The Hong Kong Hospital Authority found a drop of 20% to 30% in lung capacity in some people who recovered from the disease, and lung scans suggested organ damage.[228] This may also lead to post-intensive care syndrome following recovery.[229]
Immunity
It is unknown (as of April 2020) if past infection provides effective and long-term immunity in people who recover from the disease.[230] Cases in which recovery from COVID‑19 was followed by positive tests for coronavirus at a later date have been reported.[231] However, these cases are believed to be lingering infection rather than reinfection,[231] or false positives due to remaining RNA fragments. Some other coronaviruses circulating in people are capable of reinfection after roughly a year.[232]
History
The virus is thought to be natural and has an animal origin,[60] through spillover infection.[233] The first known human infections were in China. A study of the first 41 cases of confirmed COVID‑19, published in January 2020 in The Lancet, reported the earliest date of onset of symptoms as 1 December 2019.[234][235][236] Official publications from the WHO reported the earliest onset of symptoms as 8 December 2019.[237] Human-to-human transmission was confirmed by the WHO and Chinese authorities by 20 January 2020.[238][239] According to official Chinese sources, these were mostly linked to the Huanan Seafood Wholesale Market, which also sold live animals.[240] In May 2020, George Gao, the director of the Chinese Center for Disease Control and Prevention, said animal samples collected from the seafood market had tested negative for the virus, indicating that the market was the site of an early superspreading event, but it was not the site of the initial outbreak.[241] Traces of the virus have been found in wastewater that was collected from Milan and Turin, Italy, on 18 December 2019.[242]
There are several theories about where the very first case (the so-called patient zero) originated.[243] According to an unpublicised report from the Chinese government, the first case can be traced back to 17 November 2019; the person was a 55-year old citizen in the Hubei province. There were four men and five women reported to be infected in November, but none of them were "patient zero". By December 2019, the spread of infection was almost entirely driven by human-to-human transmission.[147][244] The number of coronavirus cases in Hubei gradually increased, reaching 60 by 20 December[245] and at least 266 by 31 December.[246] On 24 December, Wuhan Central Hospital sent a bronchoalveolar lavage fluid (BAL) sample from an unresolved clinical case to sequencing company Vision Medicals. On 27 and 28 December, Vision Medicals informed the Wuhan Central Hospital and the Chinese CDC of the results of the test, showing a new coronavirus.[247] A pneumonia cluster of unknown cause was observed on 26 December and treated by the doctor Zhang Jixian in Hubei Provincial Hospital, who informed the Wuhan Jianghan CDC on 27 December.[248] On 30 December, a test report addressed to Wuhan Central Hospital, from company CapitalBio Medlab, stated an erroneous positive result for SARS, causing a group of doctors at Wuhan Central Hospital to alert their colleagues and relevant hospital authorities of the result. That evening, the Wuhan Municipal Health Commission issued a notice to various medical institutions on "the treatment of pneumonia of unknown cause".[249] Eight of these doctors, including Li Wenliang (punished on 3 January),[250] were later admonished by the police for spreading false rumours, and another, Ai Fen, was reprimanded by her superiors for raising the alarm.[251]
The Wuhan Municipal Health Commission made the first public announcement of a pneumonia outbreak of unknown cause on 31 December, confirming 27 cases[252][253][254]—enough to trigger an investigation.[255]
During the early stages of the outbreak, the number of cases doubled approximately every seven and a half days.[256] In early and mid-January 2020, the virus spread to other Chinese provinces, helped by the Chinese New Year migration and Wuhan being a transport hub and major rail interchange.[62] On 20 January, China reported nearly 140 new cases in one day, including two people in Beijing and one in Shenzhen.[257] Later official data shows 6,174 people had already developed symptoms by then,[258] and more may have been infected.[259] A report in The Lancet on 24 January indicated human transmission, strongly recommended personal protective equipment for health workers, and said testing for the virus was essential due to its "pandemic potential".[260][261] On 30 January, the WHO declared the coronavirus a public health emergency of international concern.[259] By this time, the outbreak spread by a factor of 100 to 200 times.[262]
On 31 January 2020, Italy had its first confirmed cases, two tourists from China.[263] As of 13 March 2020, the WHO considered Europe the active centre of the pandemic.[264] On 19 March 2020, Italy overtook China as the country with the most deaths.[265] By 26 March, the United States had overtaken China and Italy with the highest number of confirmed cases in the world.[266] Research on coronavirus genomes indicates the majority of COVID‑19 cases in New York came from European travellers, rather than directly from China or any other Asian country.[267] Retesting of prior samples found a person in France who had the virus on 27 December 2019[268][269] and a person in the United States who died from the disease on 6 February 2020.[270]
On 11 June 2020, after 55 days without a locally transmitted case,[271] Beijing reported the first COVID‑19 case, followed by two more cases on 12 June.[272] By 15 June 79 cases were officially confirmed.[273] Most of these patients went to Xinfadi Wholesale Market.[271][274]
Epidemiology
Several measures are commonly used to quantify mortality.[275] These numbers vary by region and over time and are influenced by the volume of testing, healthcare system quality, treatment options, time since the initial outbreak, and population characteristics such as age, sex, and overall health.[276]
The death-to-case ratio reflects the number of deaths divided by the number of diagnosed cases within a given time interval. Based on Johns Hopkins University statistics, the global death-to-case ratio is 3.6% (767,422/21,280,608) as of 15 August 2020.[8] The number varies by region.[277]
Other measures include the case fatality rate (CFR), which reflects the percentage of diagnosed individuals who die from a disease, and the infection fatality rate (IFR), which reflects the percentage of infected individuals (diagnosed and undiagnosed) who die from a disease. These statistics are not time-bound and follow a specific population from infection through case resolution. Many academics have attempted to calculate these numbers for specific populations.[278]
Outbreaks have occurred in prisons due to crowding and an inability to enforce adequate social distancing.[279][280] In the United States, the prisoner population is aging and many of them are at high risk for poor outcomes from COVID‑19 due to high rates of coexisting heart and lung disease, and poor access to high-quality healthcare.[279]
Infection fatality rate
Infection fatality rate or infection fatality ratio (IFR) is distinguished from case fatality rate (CFR). The CFR for a disease is the proportion of deaths from the disease compared to the total number of people diagnosed with the disease (within a certain period of time). The IFR, in contrast, is the proportion of deaths among all the infected individuals. IFR, unlike CFR, attempts to account for all asymptomatic and undiagnosed infections.
Our World in Data states that, as of 25 March 2020, the IFR for coronavirus cannot be accurately calculated.[283] In February, the World Health Organization reported estimates of IFR between 0.33% and 1%.[284][285] On 2 July, The WHO's Chief Scientist reported that the average IFR estimate presented at a two-day WHO expert forum was about 0.6%.[286][287]
The CDC estimates for planning purposes that the IFR is 0.65% and that 40% of infected individuals are asymptomatic, suggesting a fatality rate among those who are symptomatic of 1.08% (.65/60) (as of 10 July).[288][289] According to the University of Oxford Centre for Evidence-Based Medicine (CEBM), random antibody testing in Germany suggested a national IFR of 0.37% (0.12% to 0.87%).[290][291][292] To get a better view on the number of people infected, as of April 2020, initial antibody testing had been carried out, but peer-reviewed scientific analyses had not yet been published.[293][294] On 1 May antibody testing in New York City suggested an IFR of 0.86%.[295]
Firm lower limits of IFRs have been established in a number of locations such as New York City and Bergamo in Italy since the IFR cannot be less than the population fatality rate. As of 10 July, in New York City, with a population of 8.4 million, 23,377 individuals (18,758 confirmed and 4,619 probable) have died with COVID‑19 (0.28% of the population).[296] In Bergamo province, 0.57% of the population has died.[297]
Sex differences
Early reviews of epidemiologic data showed greater impact of the pandemic and a higher mortality rate in men in China and Italy.[298][1][299] The Chinese Center for Disease Control and Prevention reported the death rate was 2.8% for men and 1.7% for women.[300] Later reviews in June 2020 indicated that there is no significant difference in susceptibility or in CFR between genders.[301][302] One review acknowledges the different mortality rates in Chinese men, suggesting that it may be attributable to lifestyle choices such as smoking and drinking alcohol rather than genetic factors.[303] Sex-based immunological differences, lesser prevalence of smoking in women and men developing co-morbid conditions such as hypertension at a younger age than women could have contributed to the higher mortality in men.[304] In Europe, 57% of the infected people were men and 72% of those died with COVID‑19 were men.[305] As of April 2020, the US government is not tracking sex-related data of COVID‑19 infections.[306] Research has shown that viral illnesses like Ebola, HIV, influenza and SARS affect men and women differently.[306]
| Percentage of infected people who are hospitalized | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.1 (0.07–0.2) |
0.5 (0.3–0.8) |
0.9 (0.5–1.5) |
1.3 (0.7–2.1) |
2.6 (1.5–4.2) |
5.1 (2.9–8.3) |
7.8 (4.4–12.8) |
19.3 (10.9–31.6) |
2.6 (1.5–4.3) |
| Male | 0.2 (0.08–0.2) |
0.6 (0.3–0.9) |
1.2 (0.7–1.9) |
1.6 (0.9–2.6) |
3.2 (1.8–5.2) |
6.7 (3.7–10.9) |
11.0 (6.2–17.9) |
37.6 (21.1–61.3) |
3.3 (1.8–5.3) |
| Total | 0.1 (0.08–0.2) |
0.5 (0.3–0.8) |
1.1 (0.6–1.7) |
1.4 (0.8–2.3) |
2.9 (1.6–4.7) |
5.8 (3.3–9.5) |
9.3 (5.2–15.1) |
26.2 (14.8–42.7) |
2.9 (1.7–4.8) |
| Percentage of hospitalized people who go to Intensive Care Unit | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 16.7 (14.3–19.3) |
8.7 (7.5–9.9) |
11.9 (10.9–13.0) |
16.6 (15.6–17.7) |
20.7 (19.8–21.6) |
23.1 (22.2–24.0) |
18.7 (18.0–19.5) |
4.2 (4.0–4.5) |
14.3 (13.9–14.7) |
| Male | 26.9 (23.1–31.1) |
14.0 (12.2–16.0) |
19.2 (17.6–20.9) |
26.9 (25.4–28.4) |
33.4 (32.0–34.8) |
37.3 (36.0–38.6) |
30.2 (29.1–31.3) |
6.8 (6.5–7.2) |
23.1 (22.6–23.6) |
| Total | 22.2 (19.1–25.7) |
11.6 (10.1–13.2) |
15.9 (14.5–17.3) |
22.2 (21.0–23.5) |
27.6 (26.5–28.7) |
30.8 (29.8–31.8) |
24.9 (24.1–25.8) |
5.6 (5.3–5.9) |
19.0 (18.7–19.44) |
| Percent of hospitalized people who die | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.5 (0.2–1.0) |
0.9 (0.5–1.3) |
1.5 (1.2–1.9) |
2.6 (2.3–3.0) |
5.2 (4.8–5.6) |
10.1 (9.5–10.6) |
16.7 (16.0–17.4) |
25.2 (24.4–26.0) |
14.4 (14.0–14.8) |
| Male | 0.7 (0.3–1.5) |
1.3 (0.8–1.9) |
2.2 (1.7–2.7) |
3.8 (3.3–4.4) |
7.6 (7.0–8.2) |
14.8 (14.1–15.6) |
24.6 (23.7–25.6) |
37.1 (36.1–38.2) |
21.2 (20.8–21.7) |
| Total | 0.6 (0.2–1.3) |
1.1 (0.7–1.6) |
1.9 (1.5–2.3) |
3.3 (2.9–3.8) |
6.5 (6.0–7.0) |
12.6 (12.0–13.2) |
21.0 (20.3–21.7) |
31.6 (30.9–32.4) |
18.1 (17.8–18.4) |
| Percent of infected people who die – infection fatality rate (IFR) | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.001 (<0.001–0.002) |
0.004 (0.002–0.007) |
0.01 (0.007–0.02) |
0.03 (0.02–0.06) |
0.1 (0.08–0.2) |
0.5 (0.3–0.8) |
1.3 (0.7–2.1) |
4.9 (2.7–8.0) |
0.4 (0.2–0.6) |
| Male | 0.001 (<0.001–0.003) |
0.007 (0.003–0.01) |
0.03 (0.02–0.05) |
0.06 (0.03–0.1) |
0.2 (0.1–0.4) |
1.0 (0.6–1.6) |
2.7 (1.5–1.4) |
14.0 (7.9–22.7) |
0.7 (0.4–1.1) |
| Total | 0.001 (<0.001–0.002) |
0.005 (0.003–0.01) |
0.02 (0.01–0.03) |
0.05 (0.03–0.08) |
0.2 (0.1–0.3) |
0.7 (0.4–1.2) |
1.9 (1.1–3.2) |
8.3 (4.7–13.5) |
0.5 (0.3–0.9) |
| Numbers in parentheses are 95% credible intervals for the estimates. | |||||||||
Ethnic differences
In the US, a greater proportion of deaths due to COVID‑19 have occurred among African Americans.[308] Structural factors that prevent African Americans from practicing social distancing include their concentration in crowded substandard housing and in "essential" occupations such as public transit and health care. Greater prevalence of lacking health insurance and care and of underlying conditions such as diabetes, hypertension and heart disease also increase their risk of death.[309] Similar issues affect Native American and Latino communities.[308] According to a US health policy non-profit, 34% of American Indian and Alaska Native People (AIAN) non-elderly adults are at risk of serious illness compared to 21% of white non-elderly adults.[310] The source attributes it to disproportionately high rates of many health conditions that may put them at higher risk as well as living conditions like lack of access to clean water.[311] Leaders have called for efforts to research and address the disparities.[312]
In the U.K., a greater proportion of deaths due to COVID‑19 have occurred in those of a Black, Asian, and other ethnic minority background.[313][314][315] Several factors such as poverty, poor nutrition and living in overcrowded properties, may have caused this.
Society and culture
Name
During the initial outbreak in Wuhan, China, the virus and disease were commonly referred to as "coronavirus" and "Wuhan coronavirus",[316][317][318] with the disease sometimes called "Wuhan pneumonia".[319][320] In the past, many diseases have been named after geographical locations, such as the Spanish flu,[321] Middle East Respiratory Syndrome, and Zika virus.[322]
In January 2020, the World Health Organisation recommended 2019-nCov[323] and 2019-nCoV acute respiratory disease[324] as interim names for the virus and disease per 2015 guidance and international guidelines against using geographical locations (e.g. Wuhan, China), animal species or groups of people in disease and virus names to prevent social stigma.[325][326][327]
The official names COVID‑19 and SARS-CoV-2 were issued by the WHO on 11 February 2020.[328] WHO chief Tedros Adhanom Ghebreyesus explained: CO for corona, VI for virus, D for disease and 19 for when the outbreak was first identified (31 December 2019).[329] The WHO additionally uses "the COVID‑19 virus" and "the virus responsible for COVID‑19" in public communications.[328]
Misinformation
After the initial outbreak of COVID‑19, misinformation and disinformation regarding the origin, scale, prevention, treatment, and other aspects of the disease rapidly spread online.[330][331][332]
Other health issues
The pandemic has had many impacts on global health beyond those caused by the COVID‑19 disease itself. It has led to a reduction in hospital visits for other reasons. There have been 38% fewer hospital visits for heart attack symptoms in the United States and 40% fewer in Spain.[333] The head of cardiology at the University of Arizona said, "My worry is some of these people are dying at home because they're too scared to go to the hospital."[334] There is also concern that people with strokes and appendicitis are not seeking timely treatment.[334] Shortages of medical supplies have impacted people with various conditions.[335] In several countries there has been a marked reduction of spread of sexually transmitted infections, including HIV, attributable to COVID‑19 quarantines and social distancing measures.[336][337] Similarly, in some places, rates of transmission of influenza and other respiratory viruses significantly decreased during the pandemic.[338][339][340] The pandemic has also negatively impacted mental health globally, including increased loneliness resulting from social distancing.[341]
Other animals
Humans appear to be capable of spreading the virus to some other animals. A domestic cat in Liège, Belgium, tested positive after it started showing symptoms (diarrhoea, vomiting, shortness of breath) a week later than its owner, who was also positive.[342] Tigers and lions at the Bronx Zoo in New York, United States, tested positive for the virus and showed symptoms of COVID‑19, including a dry cough and loss of appetite.[343] Minks at two farms in the Netherlands also tested positive for COVID‑19.[344]
A study on domesticated animals inoculated with the virus found that cats and ferrets appear to be "highly susceptible" to the disease, while dogs appear to be less susceptible, with lower levels of viral replication. The study failed to find evidence of viral replication in pigs, ducks, and chickens.[345]
In March 2020, researchers from the University of Hong Kong have shown that Syrian hamsters could be a model organism for COVID‑19 research.[346]
Research
No medication or vaccine is approved with the specific indication to treat the disease.[347] International research on vaccines and medicines in COVID‑19 is underway by government organisations, academic groups, and industry researchers.[348][349] In March, the World Health Organisation initiated the "Solidarity Trial" to assess the treatment effects of four existing antiviral compounds with the most promise of efficacy.[350] The World Health Organization suspended hydroxychloroquine from its global drug trials for COVID‑19 treatments on 26 May 2020 due to safety concerns. It had previously enrolled 3,500 patients from 17 countries in the Solidarity Trial.[351] France, Italy and Belgium also banned the use of hydroxychloroquine as a COVID‑19 treatment.[352]
Modelling research has been conducted with several objectives, including predictions of the dynamics of transmission,[353] diagnosis and prognosis of infection,[354] estimation of the impact of interventions,[355][356] or allocation of resources.[357] Modelling studies are mostly based on epidemiological models,[358] estimating the number of infected people over time under given conditions. Several other types of models have been developed and used during the COVID-19 including computational fluid dynamics models to study the flow physics of COVID-19,[359] retrofits of crowd movement models to study occupant exposure,[360] mobility-data based models to investigate transmission,[361] or the use of macroeconomic models to assess the economic impact of the pandemic.[362]
There has been a great deal of COVID‑19 research, involving accelerated research processes and publishing shortcuts to meet the global demand. To minimise the harm from misinformation, medical professionals and the public are advised to expect rapid changes to available information, and to be attentive to retractions and other updates.[363]
Vaccine
There is no available vaccine, but various agencies are actively developing vaccine candidates. Previous work on SARS-CoV is being used because both SARS-CoV and SARS-CoV-2 use the ACE2 receptor to enter human cells.[364] Nine vaccine platforms are being investigated (as of August 2020),[365] with 24 candidate vaccines being tested in clinical trials.[366][367] First, researchers aim to build a whole virus vaccine. The use of such inactive virus aims to elicit a prompt immune response of the human body to a new infection with COVID‑19.[368] A second strategy, subunit vaccines, aims to create a vaccine that sensitises the immune system to certain subunits of the virus.[368] In the case of SARS-CoV-2, such research focuses on the S-spike protein that helps the virus intrude the ACE2 enzyme receptor. A third strategy is that of the nucleic acid vaccines (DNA or RNA vaccines, a novel technique for creating a vaccination).[368] Fourthly, scientists are attempting to use viral vectors to deliver the SARS-CoV-2 antigen gene into the cell. These can be replicating or non-replicating.[368] Experimental vaccines from any of these strategies would have to be tested for safety and efficacy.[369]
Antibody-dependent enhancement has been suggested as a potential challenge for vaccine development for SARS-COV-2, but this is controversial.[370]
Medications
At least 29 Phase II–IV efficacy trials in COVID‑19 were concluded in March 2020, or scheduled to provide results in April from hospitals in China.[371][372] There are more than 300 active clinical trials underway as of April 2020.[115] Seven trials were evaluating already approved treatments, including four studies on hydroxychloroquine or chloroquine.[372] Repurposed antiviral drugs make up most of the research, with nine Phase III trials on remdesivir across several countries due to report by the end of April.[371][372] Other candidates in trials include vasodilators, corticosteroids, immune therapies, lipoic acid, bevacizumab, and recombinant angiotensin-converting enzyme 2.[372]
The COVID‑19 Clinical Research Coalition has goals to 1) facilitate rapid reviews of clinical trial proposals by ethics committees and national regulatory agencies, 2) fast-track approvals for the candidate therapeutic compounds, 3) ensure standardised and rapid analysis of emerging efficacy and safety data and 4) facilitate sharing of clinical trial outcomes before publication.[373][374]
Several existing medications are being evaluated for the treatment of COVID‑19,[347] including remdesivir, chloroquine, hydroxychloroquine, lopinavir/ritonavir, and lopinavir/ritonavir combined with interferon beta.[350][375] There is tentative evidence for efficacy by remdesivir, and on 1 May 2020, the United States Food and Drug Administration (FDA) gave the drug an emergency use authorization for people hospitalized with severe COVID‑19.[376] Phase III clinical trials for several drugs are underway in several countries, including the US, China, and Italy.[347][371][377]
There are mixed results as of 3 April 2020 as to the effectiveness of hydroxychloroquine as a treatment for COVID‑19, with some studies showing little or no improvement.[378][379] One study has shown an association between hydroxychloroquine or chloroquine use with higher death rates along with other side effects.[380][381] A retraction of this study by its authors was published by The Lancet on 4 June 2020.[382] The studies of chloroquine and hydroxychloroquine with or without azithromycin have major limitations that have prevented the medical community from embracing these therapies without further study.[115] On 15 June 2020, the FDA updated the fact sheets for the emergency use authorization of remdesivir to warn that using chloroquine or hydroxychloroquine with remdesivir may reduce the antiviral activity of remdesivir.[383]
In June, initial results from a randomised trial in the United Kingdom showed that dexamethasone reduced mortality by one third for patients who are critically ill on ventilators and one fifth for those receiving supplemental oxygen.[384] Because this is a well tested and widely available treatment this was welcomed by the WHO that is in the process of updating treatment guidelines to include dexamethasone or other steroids.[385][386] Based on those preliminary results, dexamethasone treatment has been recommended by the National Institutes of Health for patients with COVID‑19 who are mechanically ventilated or who require supplemental oxygen but not in patients with COVID-19 who do not require supplemental oxygen.[387]
Cytokine storm
A cytokine storm can be a complication in the later stages of severe COVID‑19. There is preliminary evidence that hydroxychloroquine may be useful in controlling cytokine storms in late-phase severe forms of the disease.[388]
Tocilizumab has been included in treatment guidelines by China's National Health Commission after a small study was completed.[389][390] It is undergoing a Phase II non-randomised trial at the national level in Italy after showing positive results in people with severe disease.[391][392] Combined with a serum ferritin blood test to identify a cytokine storm (also called cytokine storm syndrome, not to be confused with cytokine release syndrome), it is meant to counter such developments, which are thought to be the cause of death in some affected people.[393][394][395] The interleukin-6 receptor antagonist was approved by the Food and Drug Administration (FDA) to undergo a Phase III clinical trial assessing its effectiveness on COVID‑19 based on retrospective case studies for the treatment of steroid-refractory cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017.[396] To date, there is no randomised, controlled evidence that tocilizumab is an efficacious treatment for CRS. Prophylactic tocilizumab has been shown to increase serum IL-6 levels by saturating the IL-6R, driving IL-6 across the blood-brain barrier, and exacerbating neurotoxicity while having no effect on the incidence of CRS.[397]
Lenzilumab, an anti-GM-CSF monoclonal antibody, is protective in murine models for CAR T cell-induced CRS and neurotoxicity and is a viable therapeutic option due to the observed increase of pathogenic GM-CSF secreting T-cells in hospitalised patients with COVID‑19.[398]
The Feinstein Institute of Northwell Health announced in March a study on "a human antibody that may prevent the activity" of IL-6.[399]
Passive antibodies
Transferring purified and concentrated antibodies produced by the immune systems of those who have recovered from COVID‑19 to people who need them is being investigated as a non-vaccine method of passive immunisation.[400][401] The safety and effectiveness of convalescent plasma as a treatment option requires further research.[401] This strategy was tried for SARS with inconclusive results.[400] Viral neutralisation is the anticipated mechanism of action by which passive antibody therapy can mediate defence against SARS-CoV-2. The spike protein of SARS-CoV-2 is the primary target for neutralizing antibodies.[402] It has been proposed that selection of broad-neutralizing antibodies against SARS-CoV-2 and SARS-CoV might be useful for treating not only COVID‑19 but also future SARS-related CoV infections.[402] Other mechanisms, however, such as antibody-dependent cellular cytotoxicity and/or phagocytosis, may be possible.[400] Other forms of passive antibody therapy, for example, using manufactured monoclonal antibodies, are in development.[400] Production of convalescent serum, which consists of the liquid portion of the blood from recovered patients and contains antibodies specific to this virus, could be increased for quicker deployment.[403]
Laminoid antibodies
Peru announced in April 2020 that it would begin working towards creating a vaccine, with the pharmaceutical company Farvet and Universidad Peruana Cayetano Heredia (UPCH) announcing plans to jointly develop a vaccine in Chincha.[404] Peru's Experimental Station for Scientific Research and Genetic Improvement of Alpacas belonging to the Inca Group, selected on 5 June 2020 four alpacas for the development of a new vaccine that it had been developing in conjunction with Farvet and UPCH. They also indicated that alpacas have the ability to generate some types of antibodies known as "nanobodies", which are very small and have a greater potential to treat pathogens.[405] According to Andina, research from the United States, Belgium, and Chile showed that antibodies from laminoid animals could possibly be formulated into inhaler or injection treatments for those infected with coronaviruses, with Teodosio Huanca of Peru's National Institute of Agricultural Innovation (INIA) National Camelid Program stating that Peruvian camelidae share the same genetic roots and antibodies.[406]
On 7 August, the Peruvian National Institute of Health (INS) announced that it would begin the development of a possible treatment for COVID-19 utilizing "recombinant nanoantibodies" from a llama named "Tito".[407] According to the INIA, Peru holds "the only germplasm bank of South American camelids in the world, with 1,700 samples of alpacas and 1,200 of llamas".[407]
See also
- Coronavirus diseases, a group of closely related syndromes
- Coronavirus recession
- Decoding COVID-19, 2020 PBS film documentary about the 2019–2020 COVID-19 pandemic
- Disease X, a WHO term
- List of unproven methods against COVID-19
Notes
- An uncovered cough can travel up to 8.2 metres (27 feet).[19]
- The term of art used by epidemiologists is "close contact" which is defined as less than one metre (~3.3 feet) by the WHO[6] and within ~1.8 metres (six feet) by the US Centers for Disease Control and Prevention (CDC).[20]
References
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. (February 2020). "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study". Lancet. 395 (10223): 507–513. doi:10.1016/S0140-6736(20)30211-7. PMC 7135076. PMID 32007143.
- Han X, Cao Y, Jiang N, Chen Y, Alwalid O, Zhang X, et al. (March 2020). "Novel Coronavirus Pneumonia (COVID-19) Progression Course in 17 Discharged Patients: Comparison of Clinical and Thin-Section CT Features During Recovery". Clinical Infectious Diseases. 71 (15): 723–731. doi:10.1093/cid/ciaa271. PMC 7184369. PMID 32227091.
- "Special Act for Prevention, Relief and Revitalization Measures for Severe Pneumonia with Novel Pathogens–Article Content–Laws & Regulations Database of The Republic of China". law.moj.gov.tw. Retrieved 10 May 2020.
- "Covid-19". Oxford English Dictionary (3rd ed.). Oxford University Press. April 2020. Retrieved 15 April 2020. (Subscription or UK public library membership required.)
- "Symptoms of Coronavirus". U.S. Centers for Disease Control and Prevention (CDC). 13 May 2020. Archived from the original on 17 June 2020. Retrieved 18 June 2020.
- "Q&A on coronaviruses (COVID-19)". World Health Organization. 17 April 2020. Archived from the original on 14 May 2020. Retrieved 14 May 2020.
- Nussbaumer-Streit B, Mayr V, Dobrescu AI, Chapman A, Persad E, Klerings I, et al. (April 2020). "Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review". The Cochrane Database of Systematic Reviews. 4: CD013574. doi:10.1002/14651858.CD013574. PMC 7141753. PMID 32267544.
- "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)". ArcGIS. Johns Hopkins University. Retrieved 12 August 2020.
- "Coronavirus disease 2019 (COVID-19)—Symptoms and causes". Mayo Clinic. Retrieved 14 April 2020.
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. (February 2020). "The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China". International Journal of Infectious Diseases. 91: 264–266. doi:10.1016/j.ijid.2020.01.009. PMC 7128332. PMID 31953166.
- "WHO Director-General's opening remarks at the media briefing on COVID-19". World Health Organization (WHO) (Press release). 11 March 2020. Archived from the original on 11 March 2020. Retrieved 12 March 2020.
- Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, Wade RG (23 June 2020). "The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries". PloS One. 15 (6): e0234765. doi:10.1371/journal.pone.0234765. PMC 7310678. PMID 32574165. S2CID 220046286.
- Hopkins C. "Loss of sense of smell as marker of COVID-19 infection". Ear, Nose and Throat surgery body of United Kingdom. Retrieved 28 March 2020.
- Ye Q, Wang B, Mao J (June 2020). "The pathogenesis and treatment of the `Cytokine Storm' in COVID-19". The Journal of Infection. 80 (6): 607–613. doi:10.1016/j.jinf.2020.03.037. PMC 7194613. PMID 32283152.
- Murthy S, Gomersall CD, Fowler RA (March 2020). "Care for Critically Ill Patients With COVID-19". Jama. 323 (15): 1499. doi:10.1001/jama.2020.3633. PMID 32159735.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020). "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32150360. Retrieved 18 March 2020.
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. (June 2020). "COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review". Journal of the American College of Cardiology. 75 (23): 2950–2973. doi:10.1016/j.jacc.2020.04.031. PMC 7164881. PMID 32311448.
- Velavan TP, Meyer CG (March 2020). "The COVID-19 epidemic". Tropical Medicine & International Health. 25 (3): 278–280. doi:10.1111/tmi.13383. PMC 7169770. PMID 32052514.
- Bourouiba L (March 2020). "Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19". JAMA. doi:10.1001/jama.2020.4756. PMID 32215590.
- "How COVID-19 Spreads". U.S. Centers for Disease Control and Prevention (CDC). 2 April 2020. Archived from the original on 3 April 2020. Retrieved 3 April 2020.
- "Q & A on COVID-19". European Centre for Disease Prevention and Control. Retrieved 30 April 2020.
- Stadnytskyi V, Bax CE, Bax A, Anfinrud P (June 2020). "The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission". Proceedings of the National Academy of Sciences of the United States of America. 117 (22): 11875–11877. doi:10.1073/pnas.2006874117. PMC 7275719. PMID 32404416.
- "Q&A: How is COVID-19 transmitted?". World Health Organization (WHO). Retrieved 12 July 2020.
- "Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 4 March 2020. Retrieved 26 March 2020.
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (March 2020). "Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients". AJR. American Journal of Roentgenology. 215 (1): 87–93. doi:10.2214/AJR.20.23034. PMID 32174129.
- "ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection". American College of Radiology. 22 March 2020. Archived from the original on 28 March 2020.
- "Advice for public". World Health Organization (WHO). Archived from the original on 26 January 2020. Retrieved 25 February 2020.
- "Guidance on social distancing for everyone in the UK". GOV.UK. Archived from the original on 24 March 2020. Retrieved 25 March 2020.
- "Recommendations for Cloth Face Covers". U.S. Centers for Disease Control and Prevention (CDC). 3 April 2020. Retrieved 3 June 2020.
- Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ (May 2020). "Rational use of face masks in the COVID-19 pandemic". The Lancet. Respiratory Medicine. 8 (5): 434–436. doi:10.1016/S2213-2600(20)30134-X. PMC 7118603. PMID 32203710.
- Centers for Disease Control and Prevention (5 April 2020). "What to Do if You Are Sick". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 14 February 2020. Retrieved 24 April 2020.
- "When and how to use masks". World Health Organization (WHO). Archived from the original on 7 March 2020. Retrieved 24 April 2020.
- "How to Protect Yourself & Others". U.S. Centers for Disease Control and Prevention (CDC). 8 April 2020. Archived from the original on 26 February 2020. Retrieved 9 April 2020.
- "Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)". World Health Organization (WHO). Archived from the original on 31 January 2020. Retrieved 11 February 2020.
- Mahtani S, Berger M, O'Grady S, Iati M (6 February 2020). "Hundreds of evacuees to be held on bases in California; Hong Kong and Taiwan restrict travel from mainland China". The Washington Post. Archived from the original on 7 February 2020. Retrieved 11 February 2020.
- "WHO Situation Report #87" (PDF). World Health Organization (WHO). 16 April 2020.
- "Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 6 April 2020. Archived from the original on 2 March 2020. Retrieved 19 April 2020.
- Agyeman, Akosua Adom; Chin, Ken L.; Landersdorfer, Cornelia B.; Liew, Danny; Ofori-Asenso, Richard (August 2020). "Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis". Mayo Clinic Proceedings. 95 (8): 1621–1631. doi:10.1016/j.mayocp.2020.05.030. ISSN 1942-5546. PMC 7275152. PMID 32753137.
- Berlin, David A.; Gulick, Roy M.; Martinez, Fernando J. (15 May 2020). Solomon, Caren G. (ed.). "Severe Covid-19". New England Journal of Medicine: NEJMcp2009575. doi:10.1056/NEJMcp2009575. ISSN 0028-4793.
- Gandhi RT, Lynch JB, Del Rio C (April 2020). "Mild or Moderate Covid-19". New England Journal of Medicine. doi:10.1056/NEJMcp2009249. PMID 32329974.
- Wiersinga, W. Joost; Rhodes, Andrew; Cheng, Allen C.; Peacock, Sharon J.; Prescott, Hallie C. (10 July 2020). "Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review". JAMA. doi:10.1001/jama.2020.12839. ISSN 0098-7484.
- Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, Ma K (May 2020). "A Systematic Review of Asymptomatic Infections with COVID-19". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. doi:10.1016/j.jmii.2020.05.001. PMC 7227597. PMID 32425996.
|access-date=requires|url=(help) - Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. (June 2020). "Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 53 (3): 404–412. doi:10.1016/j.jmii.2020.02.012. PMC 7128959. PMID 32173241.
- Furukawa NW, Brooks JT, Sobel J (July 2020). "Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic". Emerging Infectious Diseases. 26 (7). doi:10.3201/eid2607.201595. PMC 7323549. PMID 32364890.
- Oran DP, Topol EJ (June 2020). "Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review". Annals of Internal Medicine. doi:10.7326/M20-3012. PMC 7281624. PMID 32491919.
- Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, et al. (May 2020). "High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice—Skagit County, Washington, March 2020" (PDF). MMWR Morb. Mortal. Wkly. Rep. 69 (19): 606–610. doi:10.15585/mmwr.mm6919e6. PMID 32407303. S2CID 218647339.
- Gehanno JF, Bonneterre V, Andujar P, Pairon JC, Paris C, Petit A, et al. (June 2020). "How should data on airborne transmission of SARS-CoV-2 change occupational health guidelines?". Occupational and Environmental Medicine (Letter): oemed–2020–106707. doi:10.1136/oemed-2020-106707. PMID 32606018.
- Lewis D (July 2020). "Mounting evidence suggests coronavirus is airborne - but health advice has not caught up". Nature. 583 (7817): 510–513. doi:10.1038/d41586-020-02058-1. PMID 32647382. S2CID 220470431.
- Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J (2012). "Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review". PloS One. 7 (4): e35797. Bibcode:2012PLoSO...735797T. doi:10.1371/journal.pone.0035797. PMC 3338532. PMID 22563403.
- "New coronavirus stable for hours on surfaces". National Institutes of Health. 17 March 2020. Archived from the original on 23 March 2020. Retrieved 30 April 2020.
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. (April 2020). "Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1". New England Journal of Medicine. 382 (16): 1564–1567. doi:10.1056/NEJMc2004973. PMC 7121658. PMID 32182409.
- "Household cleaners and disinfectants can cause health problems when not used properly". U.S. Centers for Disease Control and Prevention (CDC). 24 April 2020. Retrieved 6 May 2020.
- To KK, Tsang OT, Chik-Yan Yip C, Chan KH, Wu TC, Chan JM, et al. (February 2020). "Consistent detection of 2019 novel coronavirus in saliva". Clinical Infectious Diseases. Oxford University Press. 71 (15): 841–843. doi:10.1093/cid/ciaa149. PMC 7108139. PMID 32047895.
- "COVID-19 and Our Communities–ACON–We are a New South Wales based health promotion organisation specialising in HIV prevention, HIV support and lesbian, gay, bisexual, transgender and intersex (LGBTI) health". Acon.org.au. Retrieved 29 April 2020.
- "Sex and Coronavirus Disease 2019 (COVID-19)" (PDF). nyc.gov. 27 March 2020. Retrieved 29 April 2020.
- Bingmann A (22 May 2020). "Latest findings by Ulm virologists—New coronavirus detected in breast milk". Archived from the original on 7 June 2020. Retrieved 5 June 2020.
- Groß R, Conzelmann C, Müller JA, Stenger S, Steinhart K, Kirchhoff F, Münch J (May 2020). "Detection of SARS-CoV-2 in human breastmilk". Lancet. 0 (10239): 1757–1758. doi:10.1016/S0140-6736(20)31181-8. PMC 7241971. PMID 32446324.
- "Novel Coronavirus—Information for Clinicians" (PDF). Australian Government Dept of Health.
- "Outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): increased transmission beyond China—fourth update" (PDF). European Centre for Disease Prevention and Control. 14 February 2020. Retrieved 8 March 2020.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (April 2020). "The proximal origin of SARS-CoV-2". Nature Medicine. 26 (4): 450–452. doi:10.1038/s41591-020-0820-9. PMC 7095063. PMID 32284615.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. (February 2020). "A Novel Coronavirus from Patients with Pneumonia in China, 2019". New England Journal of Medicine. 382 (8): 727–733. doi:10.1056/NEJMoa2001017. PMC 7092803. PMID 31978945.
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) (PDF) (Report). World Health Organization (WHO). 24 February 2020. Archived (PDF) from the original on 29 February 2020. Retrieved 21 March 2020.
- "Coronavirus and COVID-19: What You Should Know". WebMD. Retrieved 31 July 2020.
- Verdecchia P, Cavallini C, Spanevello A, Angeli F (June 2020). "The pivotal link between ACE2 deficiency and SARS-CoV-2 infection". European Journal of Internal Medicine. 76: 14–20. doi:10.1016/j.ejim.2020.04.037. PMC 7167588. PMID 32336612.
- Letko M, Marzi A, Munster V (April 2020). "Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses". Nature Microbiology. 5 (4): 562–569. doi:10.1038/s41564-020-0688-y. PMC 7095430. PMID 32094589.
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (April 2020). "Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target". Intensive Care Medicine. 46 (4): 586–590. doi:10.1007/s00134-020-05985-9. PMC 7079879. PMID 32125455.
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. (February 2020). "High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa". International Journal of Oral Science. 12 (1): 8. doi:10.1038/s41368-020-0074-x. PMC 7039956. PMID 32094336.
- Gurwitz D (March 2020). "Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics". Drug Development Research. doi:10.1002/ddr.21656. PMC 7228359. PMID 32129518.
- Li YC, Bai WZ, Hashikawa T (February 2020). "The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients". Journal of Medical Virology. 92 (6): 552–555. doi:10.1002/jmv.25728. PMC 7228394. PMID 32104915.
- Baig AM, Khaleeq A, Ali U, Syeda H (April 2020). "Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms". ACS Chemical Neuroscience. 11 (7): 995–998. doi:10.1021/acschemneuro.0c00122. PMC 7094171. PMID 32167747.
- Gu J, Han B, Wang J (May 2020). "COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission". Gastroenterology. 158 (6): 1518–1519. doi:10.1053/j.gastro.2020.02.054. PMC 7130192. PMID 32142785.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (June 2004). "Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis". The Journal of Pathology. 203 (2): 631–7. doi:10.1002/path.1570. PMC 7167720. PMID 15141377.
- Zheng YY, Ma YT, Zhang JY, Xie X (May 2020). "COVID-19 and the cardiovascular system". Nature Reviews. Cardiology. 17 (5): 259–260. doi:10.1038/s41569-020-0360-5. PMC 7095524. PMID 32139904.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. (February 2020). "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China". JAMA. 323 (11): 1061–1069. doi:10.1001/jama.2020.1585. PMC 7042881. PMID 32031570.
- Turner AJ, Hiscox JA, Hooper NM (June 2004). "ACE2: from vasopeptidase to SARS virus receptor". Trends in Pharmacological Sciences. 25 (6): 291–4. doi:10.1016/j.tips.2004.04.001. PMC 7119032. PMID 15165741.
- Klok FA, Kruip MJ, van der Meer NJ, Arbous MS, Gommers DA, Kant KM, et al. (April 2020). "Incidence of thrombotic complications in critically ill ICU patients with COVID-19". Thrombosis Research. 191: 145–147. doi:10.1016/j.thromres.2020.04.013. PMC 7146714. PMID 32291094.
- Cui S, Chen S, Li X, Liu S, Wang F (April 2020). "Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia". Journal of Thrombosis and Haemostasis. 18 (6): 1421–1424. doi:10.1111/jth.14830. PMC 7262324. PMID 32271988.
- Wadman M (April 2020). "How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes". Science. doi:10.1126/science.abc3208.
- Coronavirus: Kidney Damage Caused by COVID-19, Johns Hopkins Medicine, C. John Sperati, updated 14 May 2020.
- Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S (May 2020). "COVID-19 Autopsies, Oklahoma, USA". American Journal of Clinical Pathology. 153 (6): 725–733. doi:10.1093/ajcp/aqaa062. PMC 7184436. PMID 32275742.
- Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (March 2020). "The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality". International Journal of Antimicrobial Agents. 55 (5): 105954. doi:10.1016/j.ijantimicag.2020.105954. PMC 7118634. PMID 32234467.
- "CDC Tests for 2019-nCoV". U.S. Centers for Disease Control and Prevention. 5 February 2020. Archived from the original on 14 February 2020. Retrieved 12 February 2020.
- "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". World Health Organization (WHO). Archived from the original on 17 March 2020. Retrieved 13 March 2020.
- "2019 Novel Coronavirus (2019-nCoV) Situation Summary". U.S. Centers for Disease Control and Prevention (CDC). 30 January 2020. Archived from the original on 26 January 2020. Retrieved 30 January 2020.
- "Real-Time RT-PCR Panel for Detection 2019-nCoV". U.S. Centers for Disease Control and Prevention (CDC). 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- "Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe". GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
- Brueck H (30 January 2020). "There's only one way to know if you have the coronavirus, and it involves machines full of spit and mucus". Business Insider. Archived from the original on 1 February 2020. Retrieved 1 February 2020.
- "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". Archived from the original on 21 February 2020. Retrieved 26 February 2020.
- Cohen J, Normile D (January 2020). "New SARS-like virus in China triggers alarm" (PDF). Science. 367 (6475): 234–235. Bibcode:2020Sci...367..234C. doi:10.1126/science.367.6475.234. PMID 31949058. S2CID 210701594. Archived (PDF) from the original on 11 February 2020. Retrieved 11 February 2020.
- "Severe acute respiratory syndrome coronavirus 2 data hub". NCBI. Archived from the original on 21 March 2020. Retrieved 4 March 2020.
- Petherick A (April 2020). "Developing antibody tests for SARS-CoV-2". Lancet. 395 (10230): 1101–1102. doi:10.1016/s0140-6736(20)30788-1. PMC 7270070. PMID 32247384.
- Vogel G (March 2020). "New blood tests for antibodies could show true scale of coronavirus pandemic". Science. doi:10.1126/science.abb8028.
- Pang J, Wang MX, Ang IY, Tan SH, Lewis RF, Chen JI, et al. (February 2020). "Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review". Journal of Clinical Medicine. 9 (3): 623. doi:10.3390/jcm9030623. PMC 7141113. PMID 32110875.
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. (June 2020). "Antibody tests for identification of current and past infection with SARS-CoV-2". The Cochrane Database of Systematic Reviews. 6: CD013652. doi:10.1002/14651858.CD013652. PMID 32584464. S2CID 220061130.
- AFP News Agency (11 April 2020). "How false negatives are complicating COVID-19 testing". Al Jazeera website Retrieved 12 April 2020.
- "Coronavirus (COVID-19) Update: FDA Issues first Emergency Use Authorization for Point of Care Diagnostic" (Press release). U.S. Food and Drug Administration (FDA). 21 March 2020. Archived from the original on 21 March 2020. Retrieved 22 March 2020.
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. (July 2020). "Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease". The Cochrane Database of Systematic Reviews. 7: CD013665. doi:10.1002/14651858.CD013665. PMID 32633856. S2CID 220384495.
- Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. (February 2020). "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)". Military Medical Research. 7 (1): 4. doi:10.1186/s40779-020-0233-6. PMC 7003341. PMID 32029004.
- Lee EY, Ng MY, Khong PL (April 2020). "COVID-19 pneumonia: what has CT taught us?". The Lancet. Infectious Diseases. 20 (4): 384–385. doi:10.1016/S1473-3099(20)30134-1. PMC 7128449. PMID 32105641. Archived from the original on 8 March 2020. Retrieved 13 March 2020.
- "ICD-10 Version:2019". World Health Organization. 2019. Archived from the original on 31 March 2020. Retrieved 31 March 2020.
U07.2—COVID-19, virus not identified—COVID-19 NOS—Use this code when COVID-19 is diagnosed clinically or epidemiologically but laboratory testing is inconclusive or not available. Use additional code, if desired, to identify pneumonia or other manifestations
- Hanley B, Lucas SB, Youd E, Swift B, Osborn M (May 2020). "Autopsy in suspected COVID-19 cases". Journal of Clinical Pathology. 73 (5): 239–242. doi:10.1136/jclinpath-2020-206522. PMID 32198191.
- Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. (March 2020). "[A pathological report of three COVID-19 cases by minimally invasive autopsies]". Zhonghua Bing Li Xue Za Zhi = Chinese Journal of Pathology (in Chinese). 49 (5): 411–417. doi:10.3760/cma.j.cn112151-20200312-00193. PMID 32172546. S2CID 212729698.
- Giani M, Seminati D, Lucchini A, Foti G, Pagni F (May 2020). "Exuberant Plasmocytosis in Bronchoalveolar Lavage Specimen of the First Patient Requiring Extracorporeal Membrane Oxygenation for SARS-CoV-2 in Europe". Journal of Thoracic Oncology. 15 (5): e65–e66. doi:10.1016/j.jtho.2020.03.008. PMC 7118681. PMID 32194247.
- Lillicrap D (April 2020). "Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia". Journal of Thrombosis and Haemostasis. 18 (4): 786–787. doi:10.1111/jth.14781. PMC 7166410. PMID 32212240.
- Mitra A, Dwyre DM, Schivo M, Thompson GR, Cohen SH, Ku N, Graff JP (March 2020). "Leukoerythroblastic reaction in a patient with COVID-19 infection". American Journal of Hematology. 95 (8): 999–1000. doi:10.1002/ajh.25793. PMC 7228283. PMID 32212392.
- Maier BF, Brockmann D (May 2020). "Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China". Science. 368 (6492): 742–746. Bibcode:2020Sci...368..742M. doi:10.1126/science.abb4557. PMC 7164388. PMID 32269067. ("...initial exponential growth expected for an unconstrained outbreak.")
- Wiles S (9 March 2020). "The three phases of Covid-19—and how we can make it manageable". The Spinoff. Archived from the original on 27 March 2020. Retrieved 9 March 2020.
- Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD (March 2020). "How will country-based mitigation measures influence the course of the COVID-19 epidemic?". Lancet. 395 (10228): 931–934. doi:10.1016/S0140-6736(20)30567-5. PMC 7158572. PMID 32164834.
A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation—e.g. minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies.
- Barclay E (10 March 2020). "How canceled events and self-quarantines save lives, in one chart". Vox. Archived from the original on 12 March 2020. Retrieved 12 March 2020.
- Barclay E, Scott D, Animashaun A (7 April 2020). "The US doesn't just need to flatten the curve. It needs to "raise the line."". Vox. Archived from the original on 7 April 2020.
- Wiles S (14 March 2020). "After 'Flatten the Curve', we must now 'Stop the Spread'. Here's what that means". The Spinoff. Archived from the original on 26 March 2020. Retrieved 13 March 2020.
- Grenfell R, Drew T (17 February 2020). "Here's Why It's Taking So Long to Develop a Vaccine for the New Coronavirus". Science Alert. Archived from the original on 28 February 2020. Retrieved 26 February 2020.
- "COVID-19 Treatment Guidelines". www.nih.gov. National Institutes of Health. Retrieved 21 April 2020.
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (April 2020). "Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review". JAMA. doi:10.1001/jama.2020.6019. PMID 32282022.
- "Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission". U.S. Centers for Disease Control and Prevention (CDC). 28 June 2020.
- Centers for Disease Control and Prevention (3 February 2020). "Coronavirus Disease 2019 (COVID-19): Prevention & Treatment". Archived from the original on 15 December 2019. Retrieved 10 February 2020.
- World Health Organization. "Advice for Public". Archived from the original on 26 January 2020. Retrieved 10 February 2020.
- "My Hand-Washing Song: Readers Offer Lyrics For A 20-Second Scrub". NPR.org. Archived from the original on 20 March 2020. Retrieved 20 March 2020.
- "Wear masks in public says WHO, in update of COVID-19 advice". Reuters. 5 June 2020. Retrieved 3 July 2020.
- "When and how to use masks". WHO. Retrieved 3 July 2020.
- "Using face masks in the community—Technical Report" (PDF). ECDC. 8 April 2020.
- "Which countries have made wearing face masks compulsory?". Al Jazeera. 20 May 2020.
- "Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 17 April 2020.
- "For different groups of people: how to choose masks". NHC.gov.cn. National Health Commission of the People's Republic of China. 7 February 2020. Retrieved 22 March 2020.
Disposable medical masks: Recommended for: · People in crowded places · Indoor working environment with a relatively dense population · People going to medical institutions · Children in kindergarten and students at school gathering to study and do other activities
- Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L (April 2020). "Face masks for the public during the covid-19 crisis". BMJ. 369: m1435. doi:10.1136/bmj.m1435. PMID 32273267. S2CID 215516381.
- "Caring for Someone Sick at Home". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 3 July 2020.
- Maragakis LL. "Coronavirus, Social Distancing and Self Quarantine". www.hopkinsmedicine.org. Johns Hopkins University. Archived from the original on 18 March 2020. Retrieved 18 March 2020.
- Parker-Pope T (19 March 2020). "Deciding How Much Distance You Should Keep". The New York Times. ISSN 0362-4331. Archived from the original on 20 March 2020. Retrieved 20 March 2020.
- Systrom K, Krieger M, O'Rourke R, Stein R, Dellaert F, Lerer A (11 April 2020). "Rt Covid-19". rt.live. Retrieved 19 April 2020. Based on Bettencourt LM, Ribeiro RM (May 2008). "Real time bayesian estimation of the epidemic potential of emerging infectious diseases". PLOS ONE. 3 (5): e2185. Bibcode:2008PLoSO...3.2185B. doi:10.1371/journal.pone.0002185. PMC 2366072. PMID 18478118.
- "WHO-recommended handrub formulations". WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. World Health Organization. 19 March 2009. Retrieved 19 March 2020.
- "Coronavirus Disease 2019 (COVID-19)—Prevention & Treatment". U.S. Centers for Disease Control and Prevention (CDC). 10 March 2020. Archived from the original on 11 March 2020. Retrieved 11 March 2020.
- "List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19)". US EPA. 13 March 2020.
- Lewis D (8 July 2020). "Mounting evidence suggests coronavirus is airborne—but health advice has not caught up". Nature. 583 (7817): 510–513. doi:10.1038/d41586-020-02058-1. PMID 32647382. S2CID 220470431.
- Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, et al. (May 2020). "Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff" (PDF). The Cochrane Database of Systematic Reviews. 5: CD011621. doi:10.1002/14651858.CD011621.pub5. PMID 32412096.
- Fisher D, Heymann D (February 2020). "Q&A: The novel coronavirus outbreak causing COVID-19". BMC Medicine. 18 (1): 57. doi:10.1186/s12916-020-01533-w. PMC 7047369. PMID 32106852.
- Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. (May 2020). "Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province". Chinese Medical Journal. 133 (9): 1025–1031. doi:10.1097/CM9.0000000000000744. PMC 7147277. PMID 32044814.
- Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B (March 2020). "Comorbidities and multi-organ injuries in the treatment of COVID-19". Lancet. 395 (10228): e52. doi:10.1016/s0140-6736(20)30558-4. PMC 7270177. PMID 32171074.
- Henry BM (April 2020). "COVID-19, ECMO, and lymphopenia: a word of caution". The Lancet. Respiratory Medicine. 8 (4): e24. doi:10.1016/s2213-2600(20)30119-3. PMC 7118650. PMID 32178774.
- Wang L, Wang Y, Ye D, Liu Q (March 2020). "Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence". International Journal of Antimicrobial Agents. 55 (6): 105948. doi:10.1016/j.ijantimicag.2020.105948. PMC 7156162. PMID 32201353. Archived from the original on 27 March 2020. Retrieved 27 March 2020.
- Wang Y, Wang Y, Chen Y, Qin Q (March 2020). "Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures". Journal of Medical Virology. n/a (n/a): 568–576. doi:10.1002/jmv.25748. PMC 7228347. PMID 32134116.
- Cheng ZJ, Shan J (April 2020). "2019 Novel coronavirus: where we are and what we know". Infection. 48 (2): 155–163. doi:10.1007/s15010-020-01401-y. PMC 7095345. PMID 32072569.
- "Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected". World Health Organization (WHO). Archived from the original on 31 January 2020. Retrieved 13 February 2020.
- Farkas J (March 2020). COVID-19—The Internet Book of Critical Care (digital) (Reference manual). USA: EMCrit. Archived from the original on 11 March 2020. Retrieved 13 March 2020.
- "COVID19—Resources for Health Care Professionals". Penn Libraries. 11 March 2020. Archived from the original on 14 March 2020. Retrieved 13 March 2020.
- Roser M, Ritchie H, Ortiz-Ospina E (4 March 2020). "Coronavirus Disease (COVID-19)". Our World in Data. Archived from the original on 19 March 2020. Retrieved 12 March 2020.
- Yanping Z, et al. (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team) (17 February 2020). "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020". China CDC Weekly. Chinese Center for Disease Control and Prevention. 2 (8): 113–122. Archived from the original on 19 February 2020. Retrieved 18 March 2020.
- 코로나바이러스감염증-19 국내 발생 현황(7월 17일, 정례브리핑) (Report) (in Korean). Korea Centers for Disease Control and Prevention. 17 July 2020. Retrieved 17 July 2020.
- Actualización nº 109. Enfermedad por el coronavirus (COVID-19) (PDF) (Report) (in Spanish). Ministerio de Sanidad, Consumo y Bienestar Social. 18 May 2020. Retrieved 20 May 2020.
- "Epidemia COVID-19—Bollettino sorveglianza integrata COVID-19" (PDF) (in Italian). Istituto Superiore di Sanità. 5 June 2020. Retrieved 10 June 2020.
- Roser M, Ritchie H, Ortiz-Ospina E (6 April 2020). "Coronavirus Disease (COVID-19)". Our World in Data. Retrieved 6 April 2020.
- Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. (April 2020). "Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review". JAMA Pediatrics. doi:10.1001/jamapediatrics.2020.1467. PMID 32320004.
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. (April 2020). "SARS-CoV-2 Infection in Children". The New England Journal of Medicine. Massachusetts Medical Society. 382 (17): 1663–1665. doi:10.1056/nejmc2005073. PMC 7121177. PMID 32187458.
- Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S (June 2020). "Epidemiology of COVID-19 Among Children in China" (PDF). Pediatrics. 145 (6): e20200702. doi:10.1542/peds.2020-0702. PMID 32179660. Archived (PDF) from the original on 17 March 2020. Retrieved 16 March 2020.
- Fang L, Karakiulakis G, Roth M (April 2020). "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?". The Lancet. Respiratory Medicine. 8 (4): e21. doi:10.1016/S0140-6736(20)30311-1. PMC 7118626. PMID 32171062.
- "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 2 March 2020. Retrieved 2 March 2020.
- Vardavas CI, Nikitara K (20 March 2020). "COVID-19 and smoking: A systematic review of the evidence". Tobacco Induced Diseases. 18 (March): 20. doi:10.18332/tid/119324. PMC 7083240. PMID 32206052.
- Engin AB, Engin ED, Engin A (August 2020). "Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking". Environmental Toxicology and Pharmacology. 78: 103411. doi:10.1016/j.etap.2020.103411. PMC 7227557. PMID 32422280.
- Tamara A, Tahapary DL (1 July 2020). "Obesity as a predictor for a poor prognosis of COVID-19: A systematic review". Diabetes & Metabolic Syndrome. 14 (4): 655–659. doi:10.1016/j.dsx.2020.05.020. PMC 7217103. PMID 32438328.
- Petrakis, Demetrios; Margină, Denisa; Tsarouhas, Konstantinos; Tekos, Fotios; Stan, Miriana; Nikitovic, Dragana; Kouretas, Demetrios; Spandidos, Demetrios; Tsatsakis, Aristidis (5 May 2020). "Obesity ‑ a risk factor for increased COVID‑19 prevalence, severity and lethality (Review)". Molecular Medicine Reports. doi:10.3892/mmr.2020.11127.
- John Parkinson (25 June 2020). "Study: Majority of Children with COVID-19 Have Mild Disease, Mortality is Rare". ContagionLive.
- "Sala de Situación COVID-19 Nuevo Coronavirus 2019 Novedades al 07/05 - 18 hs- SE 19" (PDF) (in Spanish). 7 May 2020.
- Health, Australian Government Department of (4 June 2020). "COVID-19 cases by age group and sex". Australian Government Department of Health. Retrieved 4 June 2020. Health, Australian Government Department of (4 June 2020). "COVID-19 deaths by age group and sex". Australian Government Department of Health. Retrieved 4 June 2020.
- "Coronavirus Disease 2019 (COVID-19) DAILY EPIDEMIOLOGY UPDATE Updated: 3 June, 2020, 11:00 AM ET" (PDF). Public Health Agency of Canada. 3 June 2020. Retrieved 4 June 2020.
- https://www.alberta.ca/stats/covid-19-alberta-statistics.htm
- http://www.bccdc.ca/Health-Info-Site/Documents/BC_Surveillance_Summary_June_3_2020.pdf
- "Ontario COVID-19 Data Tool". Public Health Ontario.
- "Situation of the coronavirus (COVID-19) in Québec". www.quebec.ca.
- "22° informe epidemiológico COVID-19". Ministerio de Salud – Gobierno de Chile.
- https://cdn.digital.gob.cl/public_files/Campañas/Corona-Virus/Reportes/01.06.2020_Reporte_Covid19.pdf
- Yanping Z, et al. (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team) (17 February 2020). "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020". China CDC Weekly. Chinese Center for Disease Control and Prevention. 2 (8): 113–122. Archived from the original on 19 February 2020. Retrieved 18 March 2020.
- "Coronavirus Colombia". www.ins.gov.co.
- Overvågning af COVID-19 (Report) (in Danish). Statens Serum Institut. 4 June 2020. Retrieved 4 June 2020.
- "Confirmed coronavirus cases (COVID-19) in Finland". experience.arcgis.com. THL. "Tilannekatsaus koronaviruksesta - Infektiotaudit ja rokotukset - THL". Terveyden ja hyvinvoinnin laitos.
- "Coronavirus Disease 2019 (COVID-19) Daily Situation Report of the Robert Koch Institute 05/06/2020 - UPDATED STATUS FOR GERMANY" (PDF). Robert Koch Institute.
- https://www.lgl.bayern.de/gesundheit/infektionsschutz/infektionskrankheiten_a_z/coronavirus/karte_coronavirus/index.htm
- "קורונה - משרד הבריאות". Ministry of Health (Israel). 3 May 2020. Retrieved 5 May 2020.
- "Integrated surveillance of COVID-19 in Italy" (PDF). Istituto Superiore di Sanità.
- "Coronavirus Disease (COVID-19) Situation Report in Japan". toyokeizai.net.
- COVID-19 Tablero México - CONACYT (Report) (in Spanish). Mexico City: CONACYT. 3 June 2020. Retrieved 4 June 2020.
- Epidemiologische situatie COVID-19 in Nederland 3 juni 2020 (Report) (in Dutch). Bilthoven: Rijksinstituut voor Volksgezondheid en Milie. 4 June 2020. Retrieved 4 June 2020.
- "COVID-19 Dagsrapport fredag 4. juni 2020" (PDF). Folkehelseinstituttet. 4 June 2020. Retrieved 4 June 2020.
- "COVID-19 Tracker | Department of Health website". Doh.gov.ph. 4 June 2020. Retrieved 4 June 2020.
- "NOVO CORONAVÍRUS COVID-19 RELATÓRIO DE SITUAÇÃO" (PDF) (in Portuguese). 4 June 2020. Retrieved 4 June 2020.
- "Update on Covid-19 (28th May 2020)". sacoronavirus.co.za. 29 May 2020.
- 코로나바이러스감염증-19 국내 발생 현황(7월 17일, 정례브리핑) (Report). Korea Centers for Disease Control and Prevention. 17 July 2020. Retrieved 17 July 2020.
- Actualización nº 120. Enfermedad por el coronavirus (COVID-19) (PDF) (Report) (in Spanish). Ministerio de Sanidad, Consumo y Bienestar Social. 29 May 2020. Retrieved 8 August 2020.
- "FOHM Covid-19". Public Health Agency of Sweden. 5 June 2020. Retrieved 5 June 2020.
- "Todesfälle in der Schweiz nach Altersgruppen". datawrapper.dwcdn.net. 4 June 2020. Retrieved 4 June 2020.
- "Case data | Colorado COVID-19 Updates". covid19.colorado.gov.
- "COVID-19 confirmed cases and deaths by age group | Connecticut Data". data.ct.gov. 3 June 2020. Retrieved 4 June 2020.
- https://dph.georgia.gov/covid-19-daily-status-report
- "Tableau Public". public.tableau.com.
- "COVID-19 Case Demographics - the Indiana Data Hub". hub.mph.in.gov.
- "KDPH COVID-19 Dashboard". Kygeonet.maps.arcgis.com. Retrieved 21 May 2020.
- https://coronavirus.maryland.gov Probable but not lab-confirmed deaths not included
- "COVID-19 Response Reporting". Mass.gov. 20 May 2020. Retrieved 20 May 2020.
- "Weekly COVID-19 Report 5/14/2020" (PDF). Minnesota Department of Health.
- "Coronavirus COVID-19 - Mississippi State Department of Health". msdh.ms.gov. 19 May 2020. Retrieved 20 May 2020.
- "Story Map Series". mophep.maps.arcgis.com.
- "Microsoft Power BI". app.powerbigov.us.
- "Microsoft Word - HAV Situation Report #6 07MAY19" (PDF). Retrieved 3 June 2020.
- "Oregon Health Authority | COVID-19 Updates". govstatus.egov.com.
- "Texas COVID-19 Data". Dshs.texas.gov. Retrieved 3 June 2020.
- "COVID-19 Cases in Virginia: Demographics". public.tableau.com. 20 May 2020. Retrieved 20 May 2020.
- "2019 Novel Coronavirus Outbreak (COVID-19)". Washington State Department of Health. 19 May 2020. Retrieved 20 May 2020.
- "COVID-19: Wisconsin Deaths". Wisconsin Department of Health Services. 17 April 2020.
- "WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus". World Health Organization (WHO).
- Palmieri L, Andrianou X, Barbariol P, Bella A, Bellino S, Benelli E, et al. (3 April 2020). Characteristics of COVID-19 patients dying in Italy Report based on available data on April 2nd, 2020 (PDF) (Report). Istituto Superiore di Sanità. Retrieved 3 April 2020.
- Wang W, Tang J, Wei F (April 2020). "Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China". Journal of Medical Virology. 92 (4): 441–447. doi:10.1002/jmv.25689. PMC 7167192. PMID 31994742.
- "Coronavirus Age, Sex, Demographics (COVID-19)". www.worldometers.info. Archived from the original on 27 February 2020. Retrieved 26 February 2020.
- Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. (April 2020). "Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, 1–30 March 2020". MMWR. Morbidity and Mortality Weekly Report. 69 (15): 458–464. doi:10.15585/mmwr.mm6915e3. PMID 32298251.
- "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020. Retrieved 19 June 2020.
- Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, et al. (April 2020). "The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis". Journal of Medical Virology. doi:10.1002/jmv.25889. PMC 7262275. PMID 32293753.
- "Smoking and COVID-19". World Health Organization (WHO). Retrieved 19 June 2020.
- "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 4 May 2020.
- DeRobertis J (3 May 2020). "People who use drugs are more vulnerable to coronavirus. Here's what clinics are doing to help". The Advocate (Louisiana). Retrieved 4 May 2020.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020). "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32150360. Retrieved 18 March 2020.
- Heymann DL, Shindo N, et al. (WHO Scientific and Technical Advisory Group for Infectious Hazards) (February 2020). "COVID-19: what is next for public health?". Lancet. 395 (10224): 542–545. doi:10.1016/s0140-6736(20)30374-3. PMC 7138015. PMID 32061313.
- Long B, Brady WJ, Koyfman A, Gottlieb M (July 2020). "Cardiovascular complications in COVID-19". The American Journal of Emergency Medicine. 38 (7): 1504–1507. doi:10.1016/j.ajem.2020.04.048. PMC 7165109. PMID 32317203.
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. (July 2020). "Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19)". JAMA Cardiology. doi:10.1001/jamacardio.2020.3557. PMID 32730619. Lay summary.
- Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, et al. (July 2020). "Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases". JAMA Cardiology. doi:10.1001/jamacardio.2020.3551. PMID 32730555. Lay summary.
- Xu L, Liu J, Lu M, Yang D, Zheng X (May 2020). "Liver injury during highly pathogenic human coronavirus infections". Liver International. 40 (5): 998–1004. doi:10.1111/liv.14435. PMC 7228361. PMID 32170806.
- Carod-Artal FJ (May 2020). "Neurological complications of coronavirus and COVID-19". Revista de Neurología. 70 (9): 311–322. doi:10.33588/rn.7009.2020179. PMID 32329044.
- "Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19". World Health Organization (WHO). 15 May 2020. Retrieved 20 May 2020.
- HAN Archive—00432. U.S. Centers for Disease Control and Prevention (CDC) (Report). 15 May 2020. Retrieved 20 May 2020.
- Cheung E (13 March 2020). "Some recovered Covid-19 patients may have lung damage, doctors say". South China Morning Post. Archived from the original on 15 March 2020. Retrieved 15 March 2020.
- Servick K (8 April 2020). "For survivors of severe COVID-19, beating the virus is just the beginning". Science. doi:10.1126/science.abc1486. ISSN 0036-8075.
- "BSI open letter to Government on SARS-CoV-2 outbreak response". immunology.org. British Society for Immunology. Archived from the original on 14 March 2020. Retrieved 15 March 2020.
- Omer SB, Malani P, Del Rio C (April 2020). "The COVID-19 Pandemic in the US: A Clinical Update". JAMA. doi:10.1001/jama.2020.5788. PMID 32250388.
- "What if immunity to covid-19 doesn't last?". MIT Technology Review. Retrieved 1 May 2020.
- Berger K (12 March 2020). "The Man Who Saw the Pandemic Coming". Nautilus. Archived from the original on 15 March 2020. Retrieved 16 March 2020.
- Wu YC, Chen CS, Chan YJ (March 2020). "The outbreak of COVID-19: An overview". Journal of the Chinese Medical Association. 83 (3): 217–220. doi:10.1097/JCMA.0000000000000270. PMC 7153464. PMID 32134861.
- Wang C, Horby PW, Hayden FG, Gao GF (February 2020). "A novel coronavirus outbreak of global health concern". Lancet. 395 (10223): 470–473. doi:10.1016/S0140-6736(20)30185-9. PMC 7135038. PMID 31986257.
- Cohen J (January 2020). "Wuhan seafood market may not be source of novel virus spreading globally". Science. doi:10.1126/science.abb0611.
- "Novel Coronavirus—China". World Health Organization (WHO). 12 January 2020.
- Kessler G (17 April 2020). "Trump's false claim that the WHO said the coronavirus was 'not communicable'". The Washington Post. Archived from the original on 17 April 2020. Retrieved 17 April 2020.
- Kuo L (21 January 2020). "China confirms human-to-human transmission of coronavirus". The Guardian. Retrieved 18 April 2020.
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (February 2020). "[The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]". Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi (in Chinese). 41 (2): 145–151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003. PMID 32064853. S2CID 211133882.
- Areddy, James T. (26 May 2020). "China Rules Out Animal Market and Lab as Coronavirus Origin". The Wall Street Journal. Retrieved 29 May 2020.
- Kelland, Kate (19 June 2020). "Italy sewage study suggests COVID-19 was there in December 2019". Reuters. Retrieved 23 June 2020.
- Duarte F (24 February 2020). "As the cases of coronavirus increase in China and around the world, the hunt is on to identify "patient zero"". BBC News Online. Retrieved 22 March 2020.
- Heymann DL, Shindo N (February 2020). "COVID-19: what is next for public health?". Lancet. 395 (10224): 542–545. doi:10.1016/S0140-6736(20)30374-3. PMID 32061313.
- March 2020, Jeanna Bryner-Live Science Editor-in-Chief 14. "1st known case of coronavirus traced back to November in China". livescience.com. Retrieved 31 May 2020.
- Politics, Canadian (8 April 2020). "The birth of a pandemic: How COVID-19 went from Wuhan to Toronto | National Post". Retrieved 31 May 2020.
- 高昱 (26 February 2020). "独家 | 新冠病毒基因测序溯源:警报是何时拉响的" [Exclusive | Tracing the New Coronavirus gene sequencing: when did the alarm sound]. Caixin (in Chinese). Archived from the original on 27 February 2020. Retrieved 1 March 2020.
- 路子康. "最早上报疫情的她,怎样发现这种不一样的肺炎". 中国网新闻 (in Chinese). 北京. Archived from the original on 2 March 2020. Retrieved 11 February 2020.
- "Undiagnosed pneumonia—China (HU): RFI". ProMED Mail. ProMED. Retrieved 7 May 2020.
- "'Hero who told the truth': Chinese rage over coronavirus death of whistleblower doctor". The Guardian. 7 February 2020.
- Kuo L (11 March 2020). "Coronavirus: Wuhan doctor speaks out against authorities". The Guardian. London.
- "Novel Coronavirus". World Health Organization (WHO). Archived from the original on 2 February 2020. Retrieved 6 February 2020.
- "武汉现不明原因肺炎 官方确认属实:已经做好隔离". Xinhua Net 新華網. 31 December 2019. Retrieved 31 March 2020.
- "Archived copy" 武汉市卫健委关于当前我市肺炎疫情的情况通报. WJW.Wuhan.gov.cn (in Chinese). Wuhan Municipal Health Commission. 31 December 2019. Archived from the original on 9 January 2020. Retrieved 8 February 2020.CS1 maint: archived copy as title (link)
- "Mystery pneumonia virus probed in China". BBC News Online. 3 January 2020. Archived from the original on 5 January 2020. Retrieved 29 January 2020.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. (March 2020). "Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia". New England Journal of Medicine. 382 (13): 1199–1207. doi:10.1056/NEJMoa2001316. PMC 7121484. PMID 31995857.
- "China confirms sharp rise in cases of SARS-like virus across the country". 20 January 2020. Archived from the original on 20 January 2020. Retrieved 20 January 2020.
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (17 February 2020). "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020". China CDC Weekly. 2 (8): 113–122. Retrieved 18 March 2020.
- "Flattery and foot dragging: China's influence over the WHO under scrutiny". The Globe and Mail. 25 April 2020.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (24 January 2020). "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". Lancet. 395 (10223): 497–506. doi:10.1016/S0140-6736(20)30183-5. PMC 7159299. PMID 31986264.
- Horton, Richard (18 March 2020). "Scientists have been sounding the alarm on coronavirus for months. Why did Britain fail to act?". The Guardian. Retrieved 23 April 2020.
- "China delayed releasing coronavirus info, frustrating WHO". AP NEWS. 2 June 2020. Retrieved 3 June 2020.
- "Coronavirus: Primi due casi in Italia" [Coronavirus: First two cases in Italy]. Corriere della sera (in Italian). 31 January 2020. Retrieved 31 January 2020.
- Fredericks B (13 March 2020). "WHO says Europe is new epicenter of coronavirus pandemic". New York Post. Retrieved 9 May 2020.
- "Coronavirus: Number of COVID-19 deaths in Italy surpasses China as total reaches 3,405". Sky News. Retrieved 7 May 2020.
- McNeil Jr DG (26 March 2020). "The U.S. Now Leads the World in Confirmed Coronavirus Cases". The New York Times. Retrieved 27 March 2020.
- "Studies Show N.Y. Outbreak Originated in Europe". The New York Times. 8 April 2020.
- Irish J (4 May 2020). Lough R, Graff P (eds.). "After retesting samples, French hospital discovers COVID-19 case from December". Reuters. Retrieved 4 May 2020.
- Deslandes A, Berti V, Tandjaoui-Lambotte Y, Alloui C, Carbonnelle E, Zahar JR, Brichler S, Cohen Y (3 May 2020). "SARS-COV-2 was already spreading in France in late December 2019". International Journal of Antimicrobial Agents. 55 (6): 106006. doi:10.1016/j.ijantimicag.2020.106006. PMC 7196402. PMID 32371096.
- "2 died with coronavirus weeks before 1st U.S. virus death". PBS NewsHour. 22 April 2020. Retrieved 23 April 2020.
- "Beijing Covid-19 outbreak puts food markets back in infection focus". South China Morning Post. 16 June 2020. Archived from the original on 18 June 2020. Retrieved 17 June 2020.
- "北京连续确诊3例新冠患者 新发地批发市场暂停营业". www.caixin.com. Archived from the original on 18 June 2020. Retrieved 17 June 2020.
- Gan N. "China's new coronavirus outbreak sees Beijing adopt 'wartime' measures". CNN. Archived from the original on 18 June 2020. Retrieved 17 June 2020.
- "Beijing logs record 36 COVID-19 cases, linked to market cluster". CNA. Retrieved 17 June 2020.
- Centers for Disease Control and Prevention (May 2012). "Lesson 3: Measures of Risk Section 3: Mortality Frequency Measures". Principles of Epidemiology in Public Health Practice (Third ed.). U.S. Centers for Disease Control and Prevention (CDC). No. SS1978. Archived from the original on 28 February 2020. Retrieved 28 March 2020.
- Ritchie H, Roser M (25 March 2020). Chivers T (ed.). "What do we know about the risk of dying from COVID-19?". Our World in Data. Archived from the original on 28 March 2020. Retrieved 28 March 2020.
- Lazzerini M, Putoto G (May 2020). "COVID-19 in Italy: momentous decisions and many uncertainties". The Lancet. Global Health. 8 (5): e641–e642. doi:10.1016/S2214-109X(20)30110-8. PMC 7104294. PMID 32199072.
- "What do we know about the risk of dying from COVID-19?". Our World in Data. Archived from the original on 28 March 2020. Retrieved 28 March 2020.
- Hawks L, Woolhandler S, McCormick D (April 2020). "COVID-19 in Prisons and Jails in the United States". JAMA Internal Medicine. doi:10.1001/jamainternmed.2020.1856. PMID 32343355.
- Waldstein D (6 May 2020). "To Fight Virus in Prisons, C.D.C. Suggests More Screenings". The New York Times. Retrieved 14 May 2020.
- "Total confirmed cases of COVID-19 per million people". Our World in Data. Archived from the original on 19 March 2020. Retrieved 10 April 2020.
- "Total confirmed deaths due to COVID-19 per million people". Our World in Data. Archived from the original on 19 March 2020. Retrieved 10 April 2020.
- "What do we know about the risk of dying from COVID-19?". Our World in Data. Retrieved 23 April 2020.
- "Coronavirus disease 2019 (COVID-19) Situation Report—30" (PDF). 19 February 2020. Retrieved 3 June 2020.
- "Coronavirus disease 2019 (COVID-19) Situation Report—31" (PDF). 20 February 2020. Retrieved 23 April 2020.
- McNeil Jr., Donald G. (4 July 2020). "The Pandemic's Big Mystery: How Deadly Is the Coronavirus? – Even with more than 500,000 dead worldwide, scientists are struggling to learn how often the virus kills. Here's why". The New York Times. Retrieved 6 July 2020.
- "Global Research and Innovation Forum on COVID-19: Virtual Press Conference" (PDF). World Health Organization. 2 July 2020.
- "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 22 May 2020.
- Gan N, McKeehan B (11 July 2020). "CDC now estimates that 40% of people infected with Covid-19 don't have any symptoms". CNN. Retrieved 18 July 2020.
- "Global Covid-19 Case Fatality Rates". Centre for Evidence-Based Medicine. 17 March 2020. Retrieved 10 April 2020.
- Haake D (24 April 2020). "Gangelt–A representative study on the lethality of COVID-19". Medium. Retrieved 27 April 2020.
- Vogel G (21 April 2020). "Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable". Science. doi:10.1126/science.abc3831.
- "The Coronavirus Isn't Just the Flu, Bro". www.bloomberg.com. Retrieved 26 April 2020.
- Mole B (24 April 2020). "Experts demolish studies suggesting COVID-19 is no worse than flu". Ars Technica. Retrieved 26 April 2020.
- Wilson, Linus (May 2020). "SARS-CoV-2, COVID-19, Infection Fatality Rate (IFR) Implied by the Serology, Antibody, Testing in New York City". SSRN 3590771.
- "COVID-19: Data". City of New York.
- Modi C (21 April 2020). "How deadly is COVID-19? Data Science offers answers from Italy mortality data". Medium. Retrieved 23 April 2020.
- Wenham C, Smith J, Morgan R (March 2020). "COVID-19: the gendered impacts of the outbreak". Lancet. 395 (10227): 846–848. doi:10.1016/S0140-6736(20)30526-2. PMC 7124625. PMID 32151325.
- "[The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]". Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi (in Chamorro). 41 (2): 145–151. February 2020. doi:10.3760/cma.j.issn.0254-6450.2020.02.003. PMID 32064853. S2CID 211133882.
- "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)". China CDC Weekly. 2 (8): 113–122. 1 February 2020. doi:10.46234/ccdcw2020.032. ISSN 2096-7071. Retrieved 15 June 2020.
- Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. (June 2020). "Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis". Journal of Clinical Virology. 127: 104371. doi:10.1016/j.jcv.2020.104371. PMC 7195434. PMID 32315817. Retrieved 15 June 2020.
- Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. (June 2020). "Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis". The Journal of Infection. 80 (6): 656–665. doi:10.1016/j.jinf.2020.03.041. PMC 7151416. PMID 32283155. Retrieved 15 June 2020.
- Yuki K, Fujiogi M, Koutsogiannaki S (June 2020). "COVID-19 pathophysiology: A review". Clinical Immunology. 215: 108427. doi:10.1016/j.clim.2020.108427. PMC 7169933. PMID 32325252. S2CID 216028003. Retrieved 15 June 2020.
- Rabin, Roni Caryn (20 March 2020). "In Italy, Coronavirus Takes a Higher Toll on Men". The New York Times. Retrieved 7 April 2020.
- "COVID-19 weekly surveillance report". www.euro.who.int. Retrieved 7 April 2020.
- Gupta, Alisha Haridasani (3 April 2020). "Does Covid-19 Hit Women and Men Differently? U.S. Isn't Keeping Track". The New York Times. Retrieved 7 April 2020.
- Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, et al. (July 2020). "Estimating the burden of SARS-CoV-2 in France". Science. 369 (6500): 208–211. doi:10.1126/science.abc3517. PMC 7223792. PMID 32404476.
- Dorn AV, Cooney RE, Sabin ML (April 2020). "COVID-19 exacerbating inequalities in the US". Lancet. 395 (10232): 1243–1244. doi:10.1016/S0140-6736(20)30893-X. PMC 7162639. PMID 32305087.
- Adams ML, Katz DL, Grandpre J (April 2020). "Population-Based Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States". Emerging Infectious Diseases. 26 (8): 1831–1833. doi:10.3201/eid2608.200679. PMID 32324118.
- "COVID-19 Presents Significant Risks for American Indian and Alaska Native People". 14 May 2020.
- "COVID-19 Presents Significant Risks for American Indian and Alaska Native People". 14 May 2020.
- Laurencin CT, McClinton A (April 2020). "The COVID-19 Pandemic: a Call to Action to Identify and Address Racial and Ethnic Disparities". Journal of Racial and Ethnic Health Disparities. 7 (3): 398–402. doi:10.1007/s40615-020-00756-0. PMC 7166096. PMID 32306369.
- "How coronavirus deaths in the UK compare by race and ethnicity". The Independent. 9 June 2020. Retrieved 10 June 2020.
- "Emerging findings on the impact of COVID-19 on black and minority ethnic people". The Health Foundation. Retrieved 10 June 2020.
- Butcher B, Massey J (9 June 2020). "Why are more BAME people dying from coronavirus?". BBC News Online. Retrieved 10 June 2020.
- "2nd U.S. Case Of Wuhan Coronavirus Confirmed". NPR.org. Retrieved 4 April 2020.
- McNeil Jr DG (2 February 2020). "Wuhan Coronavirus Looks Increasingly Like a Pandemic, Experts Say". The New York Times. ISSN 0362-4331. Retrieved 4 April 2020.
- Griffiths J. "Wuhan coronavirus deaths spike again as outbreak shows no signs of slowing". CNN. Retrieved 4 April 2020.
- Jiang S, Xia S, Ying T, Lu L (May 2020). "A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome". Cellular & Molecular Immunology. 17 (5): 554. doi:10.1038/s41423-020-0372-4. PMC 7091741. PMID 32024976.
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. (February 2020). "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster". Lancet. 395 (10223): 514–523. doi:10.1016/S0140-6736(20)30154-9. PMC 7159286. PMID 31986261.
- Shablovsky S (22 September 2017). "The legacy of the Spanish flu". Science. 357 (6357): 1245. Bibcode:2017Sci...357.1245S. doi:10.1126/science.aao4093. ISSN 0036-8075. S2CID 44116811.
- "Stop the coronavirus stigma now". Nature. 7 April 2020. p. 165. doi:10.1038/d41586-020-01009-0. Retrieved 16 April 2020.
- "Novel Coronavirus (2019-nCoV) SITUATION REPORT—1" (PDF). World Health Organization (WHO). 21 January 2020.
- "Novel Coronavirus(2019-nCoV) Situation Report—10" (PDF). World Health Organization (WHO). 30 January 2020.
- "Novel coronavirus named 'Covid-19': WHO". TODAYonline. Archived from the original on 21 March 2020. Retrieved 11 February 2020.
- "The coronavirus spreads racism against—and among—ethnic Chinese". The Economist. 17 February 2020. Archived from the original on 17 February 2020. Retrieved 17 February 2020.
- "World Health Organization Best Practices for the Naming of New Human Infectious Diseases" (PDF). World Health Organization (WHO). May 2015.
- "Naming the coronavirus disease (COVID-19) and the virus that causes it". World Health Organization (WHO). Archived from the original on 28 February 2020. Retrieved 13 March 2020.
- Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK—eighth update (PDF) (Report). ecdc. Archived (PDF) from the original on 14 March 2020. Retrieved 19 April 2020.
- "China coronavirus: Misinformation spreads online about origin and scale". BBC News Online. 30 January 2020. Archived from the original on 4 February 2020. Retrieved 10 February 2020.
- Taylor J (31 January 2020). "Bat soup, dodgy cures and 'diseasology': the spread of coronavirus misinformation". The Guardian. Archived from the original on 2 February 2020. Retrieved 3 February 2020.
- "Here's A Running List Of Disinformation Spreading About The Coronavirus". Buzzfeed News. Archived from the original on 6 February 2020. Retrieved 8 February 2020.
- Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, et al. (June 2020). "Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic". Journal of the American College of Cardiology. 75 (22): 2871–2872. doi:10.1016/j.jacc.2020.04.011. PMC 7151384. PMID 32283124.
- 'Where are all our patients?': Covid phobia is keeping people with serious heart symptoms away from ERs, Stat News, Usha Lee McFarling, 23 April 2020.
- Faust, Jeremy Samuel (28 April 2020). "Medication Shortages Are the Next Crisis". The Atlantic. Retrieved 17 May 2020.
- "Sexually transmitted infections surveillance reports—Reports". www.health.nsw.gov.au. Retrieved 9 May 2020.
- Wareham, Jamie. "U.K. Lockdown Has 'Broken HIV Chain' With Huge Reduction In New STI Cases". Forbes. Retrieved 9 May 2020.
- Cowling BJ, Ali ST, Ng TW, Tsang TK, Li JC, Fong MW, et al. (May 2020). "Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study". The Lancet. Public Health. 5 (5): e279–e288. doi:10.1016/S2468-2667(20)30090-6. PMC 7164922. PMID 32311320.
- Klein, Alice. "Australia sees huge decrease in flu cases due to coronavirus measures". New Scientist. Retrieved 9 May 2020.
- "Weekly U.S. Influenza Surveillance Report (FluView)". U.S. Centers for Disease Control and Prevention (CDC). 8 May 2020. Retrieved 9 May 2020.
- Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ (March 2020). "The psychological impact of quarantine and how to reduce it: rapid review of the evidence". Lancet. 395 (10227): 912–920. doi:10.1016/S0140-6736(20)30460-8. PMC 7158942. PMID 32112714. Archived from the original on 13 March 2020. Retrieved 20 March 2020.
- "Coronavirus: Belgian cat infected by owner". Brusselstimes.com. 27 March 2020. Retrieved 12 April 2020.
- Goldstein J (6 April 2020). "Bronx Zoo Tiger Is Sick With the Coronavirus". The New York Times. Retrieved 9 April 2020.
- "Coronavirus hits Netherlands farm animals as minks test positive for virus". Fox News. 26 April 2020. Retrieved 27 April 2020.
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, et al. (April 2020). "Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2". Science. 368 (6494): 1016–1020. doi:10.1126/science.abb7015. PMC 7164390. PMID 32269068.
- Chan JF, Zhang AJ, Yuan S, et al. (March 2020). "Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility" (PDF). Clinical Infectious Diseases. doi:10.1093/cid/ciaa325. ISSN 1058-4838. PMC 7184405. PMID 32215622.
- Li G, De Clercq E (March 2020). "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)". Nature Reviews. Drug Discovery. 19 (3): 149–150. doi:10.1038/d41573-020-00016-0. PMID 32127666.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W (March 2020). "COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics". Human Vaccines & Immunotherapeutics. 16 (6): 1232–1238. doi:10.1080/21645515.2020.1735227. PMC 7103671. PMID 32186952.
- Zhang L, Liu Y (May 2020). "Potential interventions for novel coronavirus in China: A systematic review". Journal of Medical Virology. 92 (5): 479–490. doi:10.1002/jmv.25707. PMC 7166986. PMID 32052466.
- Kupferschmidt K, Cohen J (22 March 2020). "WHO launches global megatrial of the four most promising coronavirus treatments". Science Magazine. doi:10.1126/science.abb8497. Retrieved 27 March 2020.
- "Citing safety concerns, the W.H.O. paused tests of a drug Trump said he had taken". The New York Times. 26 May 2020.
- "France bans use of hydroxychloroquine, drug touted by Trump, in coronavirus patients". CBS News. 27 May 2020.
- Kucharski, Adam J; Russell, Timothy W; Diamond, Charlie; Liu, Yang; Edmunds, John; Funk, Sebastian; et al. "Early dynamics of transmission and control of COVID-19: a mathematical modelling study". The Lancet Infectious Diseases. 20 (5): 553–558. doi:10.1016/S1473-3099(20)30144-4. ISSN 1473-3099. Retrieved 11 August 2020.
- Wynants, Laure; Van Calster, Ben; Collins, Gary S; Riley, Richard D; Heinze, Georg; Schuit, Ewoud; et al. "Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal". BMJ: –1328. doi:10.1136/bmj.m1328. ISSN 1756-1833. Retrieved 11 August 2020.
- Giordano, Giulia; Blanchini, Franco; Bruno, Raffaele; Colaneri, Patrizio; Di Filippo, Alessandro; Di Matteo, Angela; et al. "Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy". Nature Medicine. 26 (6): 855–860. doi:10.1038/s41591-020-0883-7. Retrieved 11 August 2020.
- Prem, Kiesha; Liu, Yang; Russell, Timothy W; Kucharski, Adam J; Eggo, Rosalind M; Davies, Nicholas; et al. "The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study". The Lancet Public Health. 5 (5): –261-e270. doi:10.1016/S2468-2667(20)30073-6. ISSN 2468-2667. Retrieved 11 August 2020.
- Emanuel, Ezekiel J.; Persad, Govind; Upshur, Ross; Thome, Beatriz; Parker, Michael; Glickman, Aaron; et al. "Fair Allocation of Scarce Medical Resources in the Time of Covid-19". New England Journal of Medicine. 382 (21): 2049–2055. doi:10.1056/NEJMsb2005114. Retrieved 11 August 2020.
- "A contribution to the mathematical theory of epidemics". Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character. 115 (772): 700–721. doi:10.1098/rspa.1927.0118. Retrieved 11 August 2020.
- Mittal, Rajat; Ni, Rui; Seo, Jung-Hee. "The flow physics of COVID-19". Journal of Fluid Mechanics. 894: –2. doi:10.1017/jfm.2020.330. Retrieved 11 August 2020.
- Ronchi, Enrico; Lovreglio, Ruggiero. "EXPOSED: An occupant exposure model for confined spaces to retrofit crowd models during a pandemic". Safety Science. 130: 104834. doi:10.1016/j.ssci.2020.104834. Retrieved 11 August 2020.
- Badr, Hamada S; Du, Hongru; Marshall, Maximilian; Dong, Ensheng; Squire, Marietta M; Gardner, Lauren M. "Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study". The Lancet Infectious Diseases: –1473309920305533. doi:10.1016/S1473-3099(20)30553-3. Retrieved 11 August 2020.
- McKibbin, Warwick; Roshen, Fernando. "The global macroeconomic impacts of COVID-19: Seven scenarios" (PDF). CAMA Working Paper. doi:10.2139/ssrn.3547729.
- Bradley-Ridout G, Fuller K, Gray M, Nekolaichuk E (9 April 2020). "Navigating the COVID-19 Evidence Landscape". University of Toronto Libraries—Gerstein Science Information Centre.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (March 2020). "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls [Internet]. StatPearls. PMID 32150360. Bookshelf ID: NBK554776.
- Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, Mayhew S (9 April 2020). "The COVID-19 vaccine development landscape". Nature Reviews Drug Discovery. 19 (5): 305–06. doi:10.1038/d41573-020-00073-5. ISSN 1474-1776. PMID 32273591. Archived from the original on 10 May 2020. Retrieved 11 April 2020.
- "COVID-19 vaccine development pipeline (Refresh URL to update)". Vaccine Centre, London School of Hygiene and Tropical Medicine. 15 July 2020. Archived from the original on 18 May 2020. Retrieved 21 July 2020.
- World Health Organization (31 July 2020). "Draft landscape of COVID-19 candidate vaccines". World Health Organization. Retrieved 9 August 2020.
- "COVID-19 vaccine and therapeutics tracker". BioRender. 29 July 2020. Retrieved 3 August 2020.
- Chen WH, Strych U, Hotez PJ, Bottazzi ME (March 2020). "The SARS-CoV-2 Vaccine Pipeline: an Overview". Current Tropical Medicine Reports. 7 (2): 61–64. doi:10.1007/s40475-020-00201-6. PMC 7094941. PMID 32219057.
- Peeples L (April 2020). "News Feature: Avoiding pitfalls in the pursuit of a COVID-19 vaccine". Proceedings of the National Academy of Sciences of the United States of America. Proceedings of the National Academy of Sciences. 117 (15): 8218–8221. doi:10.1073/pnas.2005456117. PMC 7165470. PMID 32229574.
- "COVID-19 treatment and vaccine tracker" (PDF). Milken Institute. 21 April 2020. Retrieved 21 April 2020. Lay summary.
- Koch S, Pong W (13 March 2020). "First up for COVID-19: nearly 30 clinical readouts before end of April". BioCentury Inc. Retrieved 1 April 2020.
- COVID-19 Clinical Research Coalition (April 2020). "Global coalition to accelerate COVID-19 clinical research in resource-limited settings". Lancet. 395 (10233): 1322–1325. doi:10.1016/s0140-6736(20)30798-4. PMC 7270833. PMID 32247324.
- Maguire BJ, Guérin PJ (2 April 2020). "A living systematic review protocol for COVID-19 clinical trial registrations". Wellcome Open Research. 5: 60. doi:10.12688/wellcomeopenres.15821.1. PMC 7141164. PMID 32292826.
- "UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment". UN News. 18 March 2020. Archived from the original on 23 March 2020. Retrieved 23 March 2020.
- "Coronavirus (COVI D-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment". U.S. Food and Drug Administration (FDA) (Press release). 4 May 2020. Retrieved 8 June 2020.
- Beeching NJ, Fletcher TE, Fowler R (2020). "BMJ Best Practices: COVID-19" (PDF). BMJ. Archived (PDF) from the original on 22 February 2020. Retrieved 11 March 2020.
- Seley-Radtke K (3 April 2020). "Professor of Chemistry and Biochemistry and President-Elect of the International Society for Antiviral Research, University of Maryland, Baltimore County". The Conversation. Retrieved 5 April 2020.
- Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N (June 2020). "No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection". Medecine et Maladies Infectieuses (in French). 50 (4): 384. doi:10.1016/j.medmal.2020.03.006. PMC 7195369. PMID 32240719.
- Cha AE, McGinley L. "Antimalarial drug touted by President Trump is linked to increased risk of death in coronavirus patients, study says". Washington Post. Retrieved 27 May 2020.
- Mehra MR, Desai SS, Ruschitzka F, Patel AN (May 2020). "Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis". Lancet. 0. doi:10.1016/S0140-6736(20)31180-6. PMC 7255293. PMID 32450107.
- Mehra MR, Desai SS, Ruschitzka F, Patel AN (4 June 2020). "Retraction: "Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis"". Lancet. 395 (10240): 1820glish. doi:10.1016/S0140-6736(20)31324-6. PMC 7274621. PMID 32511943.
- "Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use". U.S. Food and Drug Administration (FDA) (Press release). 15 June 2020. Retrieved 15 June 2020.

- Boseley, Sarah (16 June 202). "Recovery trial for Covid-19 treatments: what we know so far". The Guardian. Retrieved 21 June 2020.
- "WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients". World Health Organisation. 16 June 2020. Retrieved 21 June 2020.
- "Q&A: Dexamethasone and COVID-19". World Health Organization (WHO). Retrieved 12 July 2020.
- "Dexamethasone | Coronavirus Disease COVID-19". COVID-19 Treatment Guidelines. National Institutes of Health. Retrieved 12 July 2020.
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. (March 2020). "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)". Clinical Infectious Diseases. 71 (15): 732–739. doi:10.1093/cid/ciaa237. PMC 7108130. PMID 32150618.
- Liu R, Miller J (3 March 2020). "China approves use of Roche drug in battle against coronavirus complications". Reuters. Archived from the original on 12 March 2020. Retrieved 14 March 2020.
- "Effective Treatment of Severe COVID-19 Patients with Tocilizumab". ChinaXiv.org. 5 March 2020. doi:10.12074/202003.00026 (inactive 4 July 2020). Archived from the original on 19 March 2020. Retrieved 14 March 2020. Cite journal requires
|journal=(help) - Ovadia D, Agenzia Z. "COVID-19—Italy launches an independent trial on tocilizumab". Univadis from Medscape. Aptus Health. Retrieved 22 April 2020.
- "Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) (TOCIVID-19)". www.clinicaltrials.gov. Retrieved 22 April 2020.
- "How doctors can potentially significantly reduce the number of deaths from Covid-19". Vox. 12 March 2020. Archived from the original on 19 March 2020. Retrieved 14 March 2020.
- Ruan Q, Yang K, Wang W, Jiang L, Song J (March 2020). "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China". Intensive Care Medicine. 46 (5): 846–848. doi:10.1007/s00134-020-05991-x. PMC 7080116. PMID 32125452.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (March 2020). "COVID-19: consider cytokine storm syndromes and immunosuppression". Lancet. 395 (10229): 1033–1034. doi:10.1016/S0140-6736(20)30628-0. PMC 7270045. PMID 32192578.
- Slater H (26 March 2020). "FDA Approves Phase III Clinical Trial of Tocilizumab for COVID-19 Pneumonia". www.cancernetwork.com. Cancer Network. Retrieved 22 April 2020.
- Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I, et al. (2017). "Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axi-cel; KTE-C19) Treatment for Patients with Refractory,Aggressive Non-Hodgkin Lymphoma (NHL)". Blood. 130 (Supplement 1): 1547. doi:10.1182/blood.V130.Suppl_1.1547.1547 (inactive 31 July 2020).
- Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, et al. (February 2019). "GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts". Blood. 133 (7): 697–709. doi:10.1182/blood-2018-10-881722. PMC 6376281. PMID 30463995.
- "Northwell Health Initiates Clinical Trials of 2 COVID-19 Drugs". 21 March 2020. Archived from the original on 23 March 2020. Retrieved 23 March 2020.
- Casadevall A, Pirofski LA (April 2020). "The convalescent sera option for containing COVID-19". The Journal of Clinical Investigation. 130 (4): 1545–1548. doi:10.1172/JCI138003. PMC 7108922. PMID 32167489.
- Piechotta V, Chai KL, Valk SJ, Doree C, Monsef I, Wood EM, et al. (July 2020). "Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review". The Cochrane Database of Systematic Reviews. 7: CD013600. doi:10.1002/14651858.CD013600.pub2. PMID 32648959. S2CID 220471694.
- Ho M (April 2020). "Perspectives on the development of neutralizing antibodies against SARS-CoV-2". Antibody Therapeutics. 3 (2): 109–114. doi:10.1093/abt/tbaa009. PMC 7291920. PMID 32566896.
- Pearce K (13 March 2020). "Antibodies from COVID-19 survivors could be used to treat patients, protect those at risk: Infusions of antibody-laden blood have been used with reported success in prior outbreaks, including the SARS epidemic and the 1918 flu pandemic". The Hub at Johns Hopkins University. Archived from the original on 14 March 2020. Retrieved 14 March 2020.
- CORREO, NOTICIAS (8 April 2020). "Perú: Coronavirus Perú | Peruanos desarrollan vacuna contra el coronavirus e | NOTICIAS CORREO PERÚ". Correo (in Spanish). Retrieved 14 August 2020.
- Barja, Lucia (5 June 2020). "Coronavirus: Científicos comienzan a probar en alpacas una vacuna contra la COVID-19 diseñada en Perú". RPP (in Spanish). Retrieved 14 August 2020.
- "Peruvian alpaca, vicuña and guanaco antibodies could also help defeat COVID-19". Andina (in Spanish). Retrieved 14 August 2020.
- Zuta Dávila, Luis. "Coronavirus: anticuerpos de alpaca, vicuña y guanaco peruanos también evitarían enfermedad". Andina (in Spanish). Retrieved 14 August 2020.
External links
Health agencies
- Coronavirus disease (COVID-19) by the World Health Organization (WHO)
- Coronavirus 2019 (COVID-19) by the US Centers for Disease Control and Prevention (CDC)
- Coronavirus (COVID-19) by the UK National Health Service (NHS)
Directories
Medical journals
- Coronavirus Disease 2019 (COVID-19) by JAMA
- Coronavirus: News and Resources by the BMJ Publishing Group
- Novel Coronavirus Information Center by Elsevier
- COVID-19 Resource Centre by The Lancet
- SARS-CoV-2 and COVID-19 by Nature
- Coronavirus (Covid-19) by The New England Journal of Medicine
- Covid-19: Novel Coronavirus by Wiley Publishing
Treatment guidelines
- "JHMI Clinical Recommendations for Available Pharmacologic Therapies for COVID-19" (PDF). The Johns Hopkins University.
- "Bouncing Back From COVID-19: Your Guide to Restoring Movement" (PDF). The Johns Hopkins School of Medicine.
- "Guidelines on the Treatment and Management of Patients with COVID-19" (PDF). Infectious Diseases Society of America. Lay summary.
- "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines" (PDF). National Institutes of Health. Lay summary.
| Classification |
|---|




%2C_pp._113-122_(cropped).png)