Troponin I
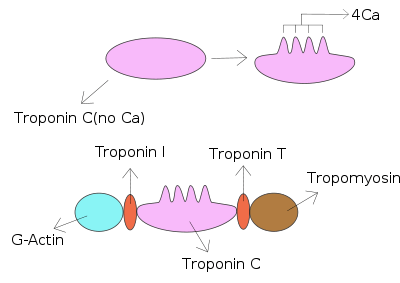
Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to actin in relaxed muscle. When calcium binds to the troponin C, it causes conformational changes which lead to dislocation of troponin I. Afterwards, tropomyosin leaves the binding site for myosin on actin leading to contraction of muscle. The letter I is given due to its inhibitory character. It is a useful marker in the laboratory diagnosis of heart attack.[1] It occurs in different plasma concentration but the same circumstances as troponin T - either test can be performed for confirmation of cardiac muscle damage and laboratories usually offer one test or the other.[2]
Three paralogs with unique tissue-specific expression patterns are expressed in humans, listed below with their locations and OMIM accessions:
cTnI
Cardiac troponin I, often denoted as cTnI, is presented in cardiac muscle tissue by a single isoform with a molecular weight of 23.9 kDa. It consists of 209 amino acid residues. The theoretical pI of cTnI is 9.05.[3] cTnI differs from other troponins due to its N-terminal extension of 26 amino acids. This extension contains two serines, residues 23 and 24, which are phosphorylated by protein kinase A in response to beta-adrenergic stimulation and important in increasing the inotropic response.[4] Phosphorylation of cTnI changes the conformation of the protein and modifies its interaction with other troponins as well as the interaction with anti-TnI antibodies. These changes alter the myofilament response to calcium, and are of interest in targeting heart failure. Multiple reaction monitoring of human cTnI has revealed that there are 14 phosphorylation sites and the pattern of phosphorylation observed these sites is changed in response to disease.[5] cTnI has been shown to be phosphorylated by protein kinase A, protein kinase C, protein kinase G, and p21-activated kinase 3.[6] A significant part of cTnI released into the patient's blood stream is phosphorylated.[7] For more than 15 years cTnI has been known as a reliable marker of cardiac muscle tissue injury. It is considered to be more sensitive and significantly more specific in diagnosis of the myocardial infarction than the "golden marker" of last decades – CK-MB, as well as total creatine kinase, myoglobin and lactate dehydrogenase isoenzymes.
Troponin I is not entirely specific for myocardial damage secondary to infarction. Other causes of raised Troponin I include chronic kidney failure, heart failure, subarachnoid haemorrhage and pulmonary embolus.[8][9]
In veterinary medicine, increased cTnI has been noted from myocardial damage after ionophore toxicity in cattle.[10]
See also
- Troponin
- Troponin T
- Troponin C
- Sliding filament model
External links
- Troponin+I at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- "Troponin". labtestsonline.
- "Troponin". labtestsonline.org/. 2019-01-09. Retrieved 2019-07-16.
- Kozlowski, LP (21 October 2016). "IPC - Isoelectric Point Calculator". Biology Direct. 11 (1): 55. doi:10.1186/s13062-016-0159-9. PMC 5075173. PMID 27769290.
- Solaro RJ, Moir AJ, Perry SV (1976). "Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart". Nature. 262 (5569): 615–616. doi:10.1038/262615a0. PMID 958429.
- Zhang P, Kirk, JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM (2012). "Multiple Reaction Monitoring to Identify Site-Specific Troponin I Phosphorylated Residues in the Failing Human Heart". Circulation. 126 (15): 1828–1837. doi:10.1161/circulationaha.112.096388. PMC 3733556. PMID 22972900.CS1 maint: multiple names: authors list (link)
- Layland J, Solaro RJ, Shah AM (2005). "Regulation of cardiac contractile function by troponin I phosphorylation". Cardiovascular Research. 66 (1): 12–21. doi:10.1016/j.cardiores.2004.12.022. PMID 15769444.
- Labugger R, Organ L, Collier C, Atar D, Van Eyk JE (2000). "Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction". Circulation. 102 (11): 1221–1226. doi:10.1161/01.cir.102.11.1221. PMID 10982534.
- Mannu GS, The non-cardiac use and significance of cardiac troponins. Scott Med J, 2014. 59(3): p. 172-8.
- Tanindi, Asil; Cemri, Mustafa (2011). "Troponin elevation in conditions other than acute coronary syndromes". Vascular Health and Risk Management. 7: 597–603. doi:10.2147/VHRM.S24509. PMC 3212425. PMID 22102783.
- https://www.frontiersin.org/articles/10.3389/fvets.2020.00531/abstract