Antibody-dependent enhancement
Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication.[1] ADE can be induced when the strength of antibody-antigen interaction is below the certain threshold.[2][3] This phenomenon might lead to both increased virus infectivity and virulence. The viruses that can cause ADE frequently share some common features such as antigenic diversity, abilities to replicate and establish persistence in immune cells.[1] ADE can occur during the development of a primary or secondary viral infection, as well as after vaccination with a subsequent virus challenge.[1][4] It has been observed mainly with positive-strand RNA viruses. Among them are Flaviviruses such as Dengue virus,[5] Yellow fever virus, Zika virus,[6][7] Coronaviruses, including alpha- and betacoronaviruses,[8][9] Orthomyxoviruses such as influenza,[10] Retroviruses such as HIV,[11] and Orthopneumoviruses such as RSV.[12][13][14]
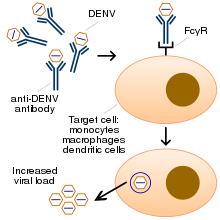
ADE occurs because some virus-specific antibodies enhance the virus entry into immune cells through interaction with Fc or/and complement receptors.[1] Cells expressing this receptor (FcγRII / CD32) are represented by monocytes, macrophages, some categories of dendritic cells and B-cells. The mechanism that involves phagocytosis of immune complexes via FcγRII / CD32 receptor is better understood.[15] Antibody-dependent enhancement can hamper vaccine development, as a vaccine may cause the production of antibodies which, via ADE, worsen the disease the vaccine is designed to protect against. This is a decisive issue during late clinical stages of vaccine development against COVID-19.[16][17] Some vaccine candidates that targeted coronaviruses, RSV virus and Dengue virus elicited ADE, and were terminated from further development.
In coronavirus infection
The phenomenon of antibody-dependent enhancement of infection has been described for alpha- and betacoronaviruses.[18][19]
Mechanism
There are various hypotheses about how ADE triggered by coronaviruses occurs, and it is likely that more than one mechanism exists. The mechanism that involves interaction of the Spike protein of coronaviruses with FcRII/CD32 receptors of the immune cells is most well supported by experimental data. The data suggest that virus-antibody/Fc-receptor complex functionally mimics viral receptor in mediating viral entry.[20]
Viral antigen
In coronaviruses ADE can be promoted by antibodies to the spike (S) protein.[8][21][19][22][23] This observation was done for alphacoronaviruses such as FIPV[23][22][24] as well as for betacoronaviruses such as SARS-CoV-1[19][25] and MERS-CoV.[20] Only antibodies targeting this protein, but not other viral proteins, are able to form complexes with coronaviruses that are phagocytosed by immune cells and provoke viral replication, instead of viral destruction. Anti-Spike immune serum increases infection of human monocyte-derived macrophages by SARS-CoV.[21] It was shown that human immunodominant SARS coronavirus epitopes trigger both enhancing and neutralizing effects in non-human primates.[19]
Cellular receptors
Experimental evidence suggests that betacoronaviruses via FcyRII/CD32 receptors can enter immune cells. The antibody-virus complex is phagocytosed by CD32 + cells after binding with FcγRII receptor.[8][26][27][21][19] It was specifically shown that the expression of two types of receptors by immune cells: FcγRIIa and FcγRIIb (but not FcγRI or FcγRIIIa) induces ADE by SARS-CoV-1.[28] Along with this finding authors of another study, while observing SARS patients, found that the severity of the disease correlates with the FcγRIIa allelic polymorphism. In patients with FcγRIIa allelic isoform that can interact with both IgG1 and IgG2, the disease is more severe compared to patients with the FcγRIIa isoform capable of binding only IgG2.[29]
Types of infected immune cells
Antibodies targeted S-protein of SARS-CoV-1 promote virus entry into CD32+ cells such as B-cells,[30][31] monocytes[26][27][25] and macrophages.[26][27][30] In these cells, the virus replicates but does not promote a productive infection. This may be due to the fact that these cells of myeloid lineage do not express enough of serine proteases required for the virion activation. However, viral replication, even without the formation of infectious virions, can lead to a massive death of immune cells bearing the Fc𝛾RIIγ receptor.
Antibodies
In some experiments it was shown that ADE was mainly caused by antibodies of the IgG2a subclass, while the tested antibodies of the IgG1 subclass did not cause such an effect.[24]
Alphacoronavirus
The feline infectious peritonitis virus (FIPV) is an alphacoronavirus that is a very common pathogen in both domestic and wild cats.[32] FIPV can cause antibody-dependent enhancement (ADE). Thus, vaccination against FIPV increases the disease seriousness.[33] It was shown that infection of macrophages by FIPV in vitro can be triggered by non-neutralising monoclonal antibodies targeting the Spike (S)-protein, and this phenomenon can also occur with diluted neutralizing antibodies.[22] ADE explains why half of cats develop peritonitis after being passively immunized with antivirus antibodies and being challenged with the same FIPV serotype.[8] In several countries an attenuated virus vaccine is available in a form of nasal drops; however, its application is still considered controversial by many experts, both in terms of safety and efficacy.[34]
Betacoronavirus
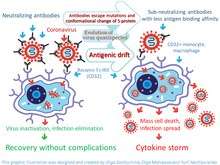
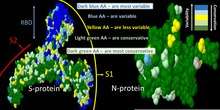
There are multiple examples of ADE triggered by betacoronaviruses. The ADE related immunopathology upon viral exposure has been a major challenge for coronavirus vaccine development[35] and may similarly impact SARS-CoV-2 vaccine research.[36] The phenomenon has been demonstrated in both cell cultures and animal models. ADE related acute lung injury has been documented in both severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) animal models.[20] It can occur during a primary infection, reinfection or infection after vaccination. For example, rabbits intranasally infected with MERS-CoV developed a pulmonary infection characterized by viremia and perivascular inflammation of the lung along with an antibody response that lacked neutralizing antibodies.[37] The rabbits produced neutralizing antibodies after the initial virus challenge, however re-exposure to MERS-CoV triggered more severe lung disease.[37] Similar observation were done with re-infected or vaccinated mice with SARS-CoV. Thus, mice were capable to develop neutralizing antibodies after re-infection with SARS-CoV itself or after vaccination with four types of vaccines. However, they all developed immunopathologic-type lung damage after SARS-CoV virus challenge, despite being protected from the virus compared to control.[35] Similar problems were observed with hamsters [31] and non-human primates. For example, vaccinated macaques due to ADE suffered from acute lung injury after the virus challenge despite having the lower viral loads after vaccination.[38][39] In these animals lung injury occurred with both type of vaccinations such as inactivated virus[38] or vector construct based on modified vaccinia Ankara virus that encoded full-length SARS-CoV spike (S) protein.[39] However, ferrets, after vaccination with a similar construct that was followed by the virus challenge developed severe hepatitis instead of a lung damage.[40]
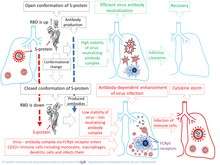
Potential linkage between pathogenesis of SARS, COVID-19 and ADE
The pathogenesis of SARS and COVID-19 diseases, may be associated with ADE, manifested in the infection of monocytes, macrophages and B-cells. Some researchers[3] believe that ADE is a key step in COVID-19 evolution from the mild to severe form with critical symptoms. ADE may explain the observed dysregulation of immune system, including apoptosis of immune cells, T-cell lymphopenia an inflammatory cascade with accumulation of macrophages and neutrophils in the lungs, and a a cytokine storm. Previously, other researchers have also put forward a similar hypothesis regarding SARS.[30][41]. ADE goes along with reduction of Th1 cytokines IL2, TNF-α and IFN-γ and increase of Th2 cytokines IL-10, IL-6, PGE-2 and INF-α, as well as with inhibition of STAT pathway.[42] This process can trigger generalized infection of immune cells in multiple organs and cytokine storm.[43][44]
Also, an ongoing question in the COVID-19 pandemic is whether—and if so, to what extent—COVID-19 receives ADE from prior infection with other coronaviruses.[45]
IgG antibodies targeting the S-protein of SARS-CoV-1
The following observations made on a small group of six patients, three of whom recovered and three died, also support the idea that antibodies to the S-protein can harm patients by causing ADE.[46] A comparative analysis of the specific humoral response showed that in patients who died from SARS-CoV-1 infection, neutralizing antibodies to the S-protein were produced much faster than in recovered people. So, it was revealed that on the 15th day of illness in patients who subsequently died, the titer of antibodies to the S-protein was significantly higher than in those who subsequently recovered. At the same time, although the titer of neutralizing antibodies during the course of the disease in patients who subsequently died grew faster than the titer in subsequently recovered patients, it also decreased faster. At the same time, in patients who subsequently recovered, the antibody titer increased more slowly, but rose to a higher level and stayed at this level longer. This dynamics of changes in antibody titers was characteristic of both IgM and IgG antibodies. It can be assumed that patients who subsequently died developed an antibody-dependent increase in viral infection in severe form and the rapid production of antibodies to the S-protein, which could not neutralize the virus, contributed to this. It is possible that the slower titer rise contributed to the production of antibodies with a higher binding constant corresponding to stronger antigen-antibody complexes, with higher affinity and avidity.[46] A significant excess of the level of antibodies in severe patients compared with non-severe patients was also observed in a sample of 325 patients in another study. [47] Other researchers received similar data on a sample of 347 SARS patients. Moreover, it was found that in patients who subsequently died, antibodies appeared first.[48]
IgG antibodies targeting the S-protein of SARS-CoV-2
Similar to SARS-CoV-1 virus results were obtained when measuring the amount of IgG antibodies to the S-protein of the SARS-CoV-2 virus found in the serum of hospitalized patients. An earlier appearance of IgG antibodies in patients with severe illness compared with those in whom it was mild was observed in a sample of 285 people.[49] Interestingly, with respect to IgM antibodies, a different dynamic was observed, they were found either in the same or in lower titer in patients with a more severe form of the disease.[49][50] In a sample of 173 patients, the effect of a significant excess of the level of antibodies in severely ill patients compared with non-severe patients was shown two weeks after symptoms onset.[51] In addition, a positive and significant correlation was found between the antibodies titer in the blood and the concentration of inflammatory markers such as C-reactive protein and lactate dehydrogenase. The data were obtained from 29 patients .[52] At the same time, a significant inverse correlation was found between the antibody titer and the number of lymphocytes.[52]
In influenza infection
Prior receipt of 2008–09 TIV (Trivalent Inactivated Influenza Vaccine) was associated with an increased risk of medically attended pH1N1 illness during the spring-summer 2009 in Canada. The occurrence of bias (selection, information) or confounding cannot be ruled out. Further experimental and epidemiological assessment is warranted. Possible biological mechanisms and immunoepidemiologic implications are considered.[53]
Natural infection and the attenuated vaccine induce antibodies that enhance the update of the homologous virus and H1N1 virus isolated several years later, demonstrating that a primary influenza A virus infection results in the induction of infection enhancing antibodies.[54]
ADE was suspected in infections with influenza A virus subtype H7N9, but knowledge is limited.
In dengue virus infection
The most widely known example of ADE occurs in the setting of infection with dengue virus, a single-stranded positive-polarity RNA virus of the family Flaviviridae. It causes a disease of varying severity in humans, from dengue fever (DF), which is usually self-limited, to dengue hemorrhagic fever and dengue shock syndrome, either of which may be life-threatening.[55] It is estimated that as many as 390 million individuals are infected with dengue virus annually.[56]
The phenomenon of ADE may be observed when a person who has previously been infected with one serotype of the dengue virus becomes infected months or years later with a different serotype. In such cases, the clinical course of the disease is more severe, and these people have higher viremia compared with those in whom ADE has not occurred. This explains the observation that while primary (first) infections cause mostly minor disease (dengue fever) in children, secondary infection (re-infection at a later date) is more likely to be associated with dengue hemorrhagic fever and/or dengue shock syndrome in both children and adults.[57]
There are four antigenically different serotypes of dengue virus (dengue virus 1–4).[58] In 2013 a fifth serotype was reported.[59] Infection with dengue virus induces the production of neutralizing homotypic immunoglobulin G (IgG) antibodies which provide lifelong immunity against the infecting serotype. Infection with dengue virus also produces some degree of cross-protective immunity against the other three serotypes.[60] Neutralizing heterotypic (cross-reactive) IgG antibodies are responsible for this cross-protective immunity, which typically persists for a period of several months to a few years. These heterotypic antibody titers decrease over long time periods (4 to 20 years).[61] While heterotypic IgG antibody titers decrease, homotypic IgG antibody titers increase over long time periods. This could be due to the preferential survival of long-lived memory B cells producing homotypic antibodies.[61]
In addition to inducing neutralizing heterotypic antibodies, infection with the dengue virus can also induce heterotypic antibodies that neutralize the virus only partially or not at all.[62] The production of such cross-reactive but non-neutralizing antibodies could be the reason for more severe secondary infections. It is thought that by binding to but not neutralizing the virus, these antibodies cause it to behave as a "trojan horse",[63][64][65] where it is delivered into the wrong compartment of dendritic cells that have ingested the virus for destruction.[66][67] Once inside the white blood cell, the virus replicates undetected, eventually generating very high virus titers which cause severe disease.[68]
A study conducted by Modhiran et al.[69] attempted to explain how non-neutralizing antibodies down-regulate the immune response in the host cell through the Toll-like receptor signaling pathway. Toll-like receptors are known to recognize extra- and intracellular viral particles and to be a major basis of the cytokines production. In vitro experiments showed that the inflammatory cytokines and type 1 interferon production were reduced when the ADE-dengue virus complex bound to the Fc receptor of THP-1 cells. This can be explained by both a decrease of Toll-like receptor production and a modification of its signaling pathway. On one hand, an unknown protein induced by the stimulated Fc receptor reduces the Toll-like receptor transcription and translation, which reduces the capacity of the cell to detect viral proteins. On the other hand, many proteins (TRIF, TRAF6, TRAM, TIRAP, IKKα, TAB1, TAB2, NF-κB complex) involved in the Toll-like receptor signaling pathway are down-regulated, which led to a decrease of the cytokine production. Two of them, TRIF and TRAF6, are respectively down-regulated by 2 proteins SARM and TANK up-regulated by the stimulated Fc receptors.
To illustrate the phenomenon of ADE, consider the following example: an epidemic of dengue fever occurred in Cuba, lasting from 1977 to 1979. The infecting serotype was dengue virus-1. This epidemic was followed by two more outbreaks of dengue fever—one in 1981 and one in 1997; dengue virus-2 was the infecting serotype in both of these later epidemics. 205 cases of dengue hemorrhagic fever and dengue shock syndrome occurred during the 1997 outbreak, all in people older than 15 years. All but three of these cases were demonstrated to have been previously infected by the dengue virus-1 serotype during the epidemic of 1977–1979.[70] Furthermore, people who had been infected with dengue virus-1 during the 1977-79 outbreak and secondarily infected with dengue virus-2 in 1997 had a 3-4 fold increased probability of developing severe disease than those secondarily infected with dengue virus-2 in 1981.[61] This scenario can be explained by the presence of neutralizing heterotypic IgG antibodies in sufficient titers in 1981, the titers of which had decreased by 1997 to the point where they no longer provided significant cross-protective immunity.
In HIV-1 virus infection
ADE of infection has also been reported in HIV. Like dengue virus, non-neutralizing level of antibodies have been found to enhance the viral infection through interactions of the complement system and receptors.[71] The increase in infection has been reported to be over 350 fold which is comparable to ADE in other viruses like dengue virus.[71] ADE in HIV can be complement-mediated or Fc receptor-mediated. Complements in the presence of HIV-1 positive sera have been found to enhance the infection of MT-2 T-cell line. The Fc-receptor mediated enhancement was reported when HIV infection was enhanced by sera from HIV-1 positive guinea pig enhanced the infection of peripheral blood mononuclear cells without the presence of any complements.[72] Complement component receptors CR2, CR3 and CR4 have been found to mediate this Complement-mediated enhancement of infection.[71][73] The infection of HIV-1 leads to activation of complements. Fragments of these complements can assist viruses with infection by facilitating viral interactions with host cells that express complement receptors.[74] The deposition of complement on the virus brings the gp120 protein close to CD4 molecules on the surface of the cells, thus leading to facilitated viral entry.[74] Viruses pre-exposed to non-neutralizing complement system have also been found to enhance infections in interdigitating dendritic cells. Opsonized viruses have not only shown enhanced entry but also favorable signaling cascades for HIV replication in interdigitating dendritic cells.[75]
HIV-1 has also shown enhancement of infection in HT-29 cells when the viruses were pre-opsonized with complements C3 and C9 in seminal fluid. This enhanced rate of infection was almost 2 times greater than infection of HT-29 cells with virus alone.[76] Subramanian et al., reported that almost 72% of serum samples out of 39 HIV positive individuals contained complements that were known to enhance the infection. They also suggested that the presence of neutralizing antibody or antibody-dependent cellular cytotoxicity-mediating antibodies in the serum contains infection-enhancing antibodies.[77] The balance between the neutralizing antibodies and infection-enhancing antibodies changes as the disease progresses. During advanced stages of the disease the proportion of infection-enhancing antibodies are generally higher than neutralizing antibodies.[78] Increase in viral protein synthesis and RNA production have been reported to occur during the complement-mediated enhancement of infection. Cells that are challenged with non-neutralizing levels of complements have been found have accelerated release of reverse transcriptase and the viral progeny.[79] The interaction of anti-HIV antibodies with non-neutralizing complement exposed viruses also aid in binding of the virus and the erythrocytes which can lead to more efficient delivery of viruses to the immune-compromised organs.[73]
ADE in HIV has raised questions about the risk of infections to volunteers who have taken sub-neutralizing levels of vaccine just like any other viruses that exhibit ADE. Gilbert et al., in 2005 reported that there was no ADE of infection when they used rgp120 vaccine in phase 1 and 2 trials.[80] It has been emphasized that much research needs to be done in the field of the immune response to HIV-1, information from these studies can be used to produce a more effective vaccine.
Mechanism
There are several possibilities to explain the phenomenon:
- A viral surface protein studded with antibodies against a virus of one serotype binds to a similar virus with a different serotype. The binding is meant to neutralize the virus surface protein from attaching to the cell, but the virus-antibody complex also binds to the Fc-region antibody receptor (FcγR) on the cell membrane. This brings the virus into close proximity to the virus-specific receptor, and the cell internalizes the virus through the normal infection route.[81]
- A virus surface protein may be attached to antibodies of a different serotype, activating the classical pathway of the complement system. The complement cascade system instead binds C1Q complex attached to the virus surface protein via the antibodies, which in turn bind C1q receptor found on cells, bringing the virus and the cell close enough for a specific virus receptor to bind the virus, beginning infection. This mechanism has not been shown specifically for dengue virus infection, but may occur with Ebola virus infection in vitro.[82]
- When an antibody to a virus is present for a different serotype, it is unable to neutralize the virus, which is then ingested into the cell as a sub-neutralized virus particle. These viruses are phagocytosed as antigen-antibody complexes, and degraded by macrophages. Upon ingestion the antibodies no longer even sub-neutralize the body due to the denaturing condition at the step for acidification of phagosome before fusion with lysosome. The virus becomes active and begins its proliferation within the cell.
See also
- Original antigenic sin
- Other ways in which antibodies can (unusually) make an infection worse instead of better
- Blocking antibody, which can be either good or bad, depending on circumstances
- Hook effect, most relevant to in vitro tests but known to have some in vivo relevances
References
- Tirado SM, Yoon KJ (2003). "Antibody-dependent enhancement of virus infection and disease". Viral Immunology. 16 (1): 69–86. doi:10.1089/088282403763635465. PMID 12725690.
- Iwasaki A, Yang Y (June 2020). "The potential danger of suboptimal antibody responses in COVID-19". Nature Reviews. Immunology. 20 (6): 339–341. doi:10.1038/s41577-020-0321-6. PMC 7187142. PMID 32317716.
- Ricke D, Malone RW (2020). "Medical Countermeasures Analysis of 2019-nCoV and Vaccine Risks for Antibody-Dependent Enhancement (ADE)". SSRN Working Paper Series. doi:10.2139/ssrn.3546070. ISSN 1556-5068.
- Tay MZ, Wiehe K, Pollara J (2019). "Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses". Frontiers in Immunology. 10: 332. doi:10.3389/fimmu.2019.00332. PMC 6404786. PMID 30873178.
- de Alwis R, Williams KL, Schmid MA, Lai CY, Patel B, Smith SA, et al. (October 2014). "Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera". PLoS Pathogens. 10 (10): e1004386. doi:10.1371/journal.ppat.1004386. PMC 4183589. PMID 25275316.
- Khandia R, Munjal A, Dhama K, Karthik K, Tiwari R, Malik YS, et al. (2018). "Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection". Frontiers in Immunology. 9: 597. doi:10.3389/fimmu.2018.00597. PMC 5925603. PMID 29740424.
- Plotkin S, Orenstein W (2012). "Yellow fever vaccine". Vaccines (6 ed.). Amsterdam: Elsevier. pp. 870–968. ISBN 9781455700905.
- Takano T, Yamada S, Doki T, Hohdatsu T (June 2019). "Pathogenesis of oral type I feline infectious peritonitis virus (FIPV) infection: Antibody-dependent enhancement infection of cats with type I FIPV via the oral route". The Journal of Veterinary Medical Science. 81 (6): 911–915. doi:10.1292/jvms.18-0702. PMC 6612493. PMID 31019150.
- Yip MS, Leung NH, Cheung CY, Li PH, Lee HH, Daëron M, et al. (May 2014). "Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus". Virology Journal. 11 (1): 82. doi:10.1186/1743-422X-11-82. PMC 4018502. PMID 24885320.
- Winarski KL, Tang J, Klenow L, Lee J, Coyle EM, Manischewitz J, et al. (July 2019). "Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics". Proceedings of the National Academy of Sciences of the United States of America. 116 (30): 15194–15199. doi:10.1073/pnas.1821317116. PMC 6660725. PMID 31296560.
- Füst G (1997). "Enhancing antibodies in HIV infection". Parasitology. 115 Suppl (7): S127-40. doi:10.1017/s0031182097001819. PMID 9571698.
- van Erp EA, van Kasteren PB, Guichelaar T, Ahout IM, de Haan CA, Luytjes W, et al. (November 2017). "In Vitro Enhancement of Respiratory Syncytial Virus Infection by Maternal Antibodies Does Not Explain Disease Severity in Infants". Journal of Virology. 91 (21). doi:10.1128/JVI.00851-17. PMC 5640862. PMID 28794038.
- Osiowy C, Horne D, Anderson R (November 1994). "Antibody-dependent enhancement of respiratory syncytial virus infection by sera from young infants". Clinical and Diagnostic Laboratory Immunology. 1 (6): 670–7. doi:10.1128/CDLI.1.6.670-677.1994. PMC 368388. PMID 8556519.
- Gimenez HB, Chisholm S, Dornan J, Cash P (May 1996). "Neutralizing and enhancing activities of human respiratory syncytial virus-specific antibodies". Clinical and Diagnostic Laboratory Immunology. 3 (3): 280–6. doi:10.1128/CDLI.3.3.280-286.1996. PMC 170331. PMID 8705669.
- Bournazos S, Gupta A, Ravetch JV (August 2020). "The role of IgG Fc receptors in antibody-dependent enhancement". Nature Reviews. Immunology: 1–11. doi:10.1038/s41577-020-00410-0. PMID 32782358.
- Thomas N (22 July 2020). "Phase 3 trials will watch for possibility of vaccine-induced enhancement of infection, Fauci says". CNN.
- Peeples L (April 2020). "News Feature: Avoiding pitfalls in the pursuit of a COVID-19 vaccine". Proceedings of the National Academy of Sciences of the United States of America. 117 (15): 8218–8221. doi:10.1073/pnas.2005456117. PMC 7165470. PMID 32229574.
- Jaume M, Yip MS, Kam YW, Cheung CY, Kien F, Roberts A, et al. (February 2012). "SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement". Hong Kong Medical Journal = Xianggang Yi Xue Za Zhi. 18 Suppl 2: 31–6. PMID 22311359.
- Wang Q, Zhang L, Kuwahara K, Li L, Liu Z, Li T, et al. (May 2016). "Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates". ACS Infectious Diseases. 2 (5): 361–76. doi:10.1021/acsinfecdis.6b00006. PMC 7075522. PMID 27627203.
- Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, et al. (February 2020). "Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry". Journal of Virology. 94 (5). doi:10.1128/JVI.02015-19. PMC 7022351. PMID 31826992.
- Hiu-lan NL (2012). "Mechanism of antibody-dependent enhancement in severe acute respiratory syndrome coronavirus infection". The University of Hong Kong Libraries. doi:10.5353/th_b4732706. Cite journal requires
|journal=(help) - Hohdatsu T, Nakamura M, Ishizuka Y, Yamada H, Koyama H (September 1991). "A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies". Archives of Virology. 120 (3–4): 207–17. doi:10.1007/bf01310476. PMC 7087175. PMID 1659798.
- Hohdatsu T, Okada S, Koyama H (1991). "Characterization of monoclonal antibodies against feline infectious peritonitis virus type II and antigenic relationship between feline, porcine, and canine coronaviruses". Archives of Virology. 117 (1–2): 85–95. doi:10.1007/BF01310494. PMC 7086586. PMID 1706593.
- Corapi WV, Olsen CW, Scott FW (November 1992). "Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus". Journal of Virology. 66 (11): 6695–705. doi:10.1128/JVI.66.11.6695-6705.1992. PMC 240165. PMID 1383568.
- Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, Shen CW, et al. (August 2014). "Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins". Biochemical and Biophysical Research Communications. 451 (2): 208–14. doi:10.1016/j.bbrc.2014.07.090. PMC 7092860. PMID 25073113.
- Li L, Wo J, Shao J, Zhu H, Wu N, Li M, et al. (December 2003). "SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients". Journal of Clinical Virology. 28 (3): 239–44. doi:10.1016/s1386-6532(03)00195-1. PMC 7128964. PMID 14522061.
- Yilla M, Harcourt BH, Hickman CJ, McGrew M, Tamin A, Goldsmith CS, et al. (January 2005). "SARS-coronavirus replication in human peripheral monocytes/macrophages". Virus Research (Virus Research ed.). 107 (1): 93–101. doi:10.1016/j.virusres.2004.09.004. PMID 15567038. S2CID 44836086.
- Jaume M, Yip MS, Cheung CY, Leung HL, Li PH, Kien F, et al. (October 2011). "Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway". Journal of Virology. 85 (20): 10582–97. doi:10.1128/JVI.00671-11. PMC 3187504. PMID 21775467.
- Yuan FF, Tanner J, Chan PK, Biffin S, Dyer WB, Geczy AF, et al. (October 2005). "Influence of FcgammaRIIA and MBL polymorphisms on severe acute respiratory syndrome". Tissue Antigens. 66 (4): 291–6. doi:10.1111/j.1399-0039.2005.00476.x. PMC 7190181. PMID 16185324.
- Yip MS, Cheung CY, Li PH, Bruzzone R, Peiris JM, Jaume M (January 2011). "Investigation of Antibody-Dependent Enhancement (ADE) of SARS coronavirus infection and its role in pathogenesis of SARS". BMC Proceedings (BMC Proceedings ed.). 5 (S1): P80. doi:10.1186/1753-6561-5-s1-p80. PMC 3019510.
- Kam YW, Kien F, Roberts A, Cheung YC, Lamirande EW, Vogel L, et al. (January 2007). "Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro". Vaccine (Vaccine ed.). 25 (4): 729–40. doi:10.1016/j.vaccine.2006.08.011. PMC 7115629. PMID 17049691.
- Vennema H, Poland A, Foley J, Pedersen NC (March 1998). "Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses". Virology. 243 (1): 150–7. doi:10.1006/viro.1998.9045. PMC 7131759. PMID 9527924.
- Vennema H, de Groot RJ, Harbour DA, Dalderup M, Gruffydd-Jones T, Horzinek MC, Spaan WJ (March 1990). "Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization". Journal of Virology. 64 (3): 1407–9. doi:10.1128/jvi.64.3.1407-1409.1990. PMC 249267. PMID 2154621.
- Negro F (April 2020). "Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis?". Swiss Medical Weekly. 150: w20249. doi:10.4414/smw.2020.20249. PMID 32298458.
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, et al. (2012). "Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus". PLOS ONE. 7 (4): e35421. Bibcode:2012PLoSO...735421T. doi:10.1371/journal.pone.0035421. PMC 3335060. PMID 22536382.
- Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, et al. (July 2020). "A perspective on potential antibody-dependent enhancement of SARS-CoV-2". Nature: 1–11. doi:10.1038/s41586-020-2538-8. PMID 32659783. S2CID 220509274.
- Houser KV, Broadbent AJ, Gretebeck L, Vogel L, Lamirande EW, Sutton T, et al. (August 2017). "Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody". PLOS Pathogens. 13 (8): e1006565. doi:10.1371/journal.ppat.1006565. PMC 5574614. PMID 28817732.
- Luo F, Liao FL, Wang H, Tang HB, Yang ZQ, Hou W (April 2018). "Evaluation of Antibody-Dependent Enhancement of SARS-CoV Infection in Rhesus Macaques Immunized with an Inactivated SARS-CoV Vaccine". Virologica Sinica. 33 (2): 201–204. doi:10.1007/s12250-018-0009-2. PMC 6178114. PMID 29541941.
- Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, et al. (February 2019). "Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection". JCI Insight. 4 (4). doi:10.1172/jci.insight.123158. PMC 6478436. PMID 30830861.
- Weingartl H, Czub M, Czub S, Neufeld J, Marszal P, Gren J, et al. (November 2004). "Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets". Journal of Virology. 78 (22): 12672–6. doi:10.1128/JVI.78.22.12672-12676.2004. PMC 525089. PMID 15507655.
- Perlman S, Dandekar AA (December 2005). "Immunopathogenesis of coronavirus infections: implications for SARS". Nature Reviews. Immunology (Nature Reviews Immunology ed.). 5 (12): 917–27. doi:10.1038/nri1732. PMC 7097326. PMID 16322745.
- Smatti MK, Al Thani AA, Yassine HM (2018-12-05). "Viral-Induced Enhanced Disease Illness". Frontiers in Microbiology. 9: 2991. doi:10.3389/fmicb.2018.02991. PMC 6290032. PMID 30568643.
- Gu J, Taylor CR (December 2003). "Acute immunodeficiency, multiple organ injury, and the pathogenesis of SARS". Applied Immunohistochemistry & Molecular Morphology. 11 (4): 281–2. doi:10.1097/00129039-200312000-00001. PMID 14663354.
- Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. (August 2005). "Multiple organ infection and the pathogenesis of SARS". The Journal of Experimental Medicine. 202 (3): 415–24. doi:10.1084/jem.20050828. PMC 2213088. PMID 16043521.
- Tetro JA (March 2020). "Is COVID-19 receiving ADE from other coronaviruses?". Microbes and Infection. 22 (2): 72–73. doi:10.1016/j.micinf.2020.02.006. PMC 7102551. PMID 32092539.
- Zhang L, Zhang F, Yu W, He T, Yu J, Yi CE, et al. (January 2006). "Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals". Journal of Medical Virology (Journal of Medical Virology ed.). 78 (1): 1–8. doi:10.1002/jmv.20499. PMC 7166884. PMID 16299724.
- Lee N, Chan PK, Ip M, Wong E, Ho J, Ho C, et al. (February 2006). "Anti-SARS-CoV IgG response in relation to disease severity of severe acute respiratory syndrome". Journal of Clinical Virology (Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology ed.). 35 (2): 179–84. doi:10.1016/j.jcv.2005.07.005. PMC 7108264. PMID 16112612.
- Ho MS, Chen WJ, Chen HY, Lin SF, Wang MC, Di J, et al. (November 2005). "Neutralizing antibody response and SARS severity". Emerging Infectious Diseases (Emerging Infectious Diseases ed.). 11 (11): 1730–7. doi:10.3201/eid1111.040659. PMC 3367364. PMID 16318725.
- Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. (June 2020). "Antibody responses to SARS-CoV-2 in patients with COVID-19". Nature Medicine (Nature Medicine ed.). 26 (6): 845–848. doi:10.1038/s41591-020-0897-1. PMID 32350462. S2CID 216609402.
- Shen L, Wang C, Zhao J, Tang X, Shen Y, Lu M, et al. (December 2020). "Delayed specific IgM antibody responses observed among COVID-19 patients with severe progression". Emerging Microbes & Infections (Emerging Microbes & Infections ed.). 9 (1): 1096–1101. doi:10.1080/22221751.2020.1766382. PMID 32476607. S2CID 219169058.
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. (March 2020). "Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019". Clinical Infectious Diseases (Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America ed.): ciaa344. doi:10.1093/cid/ciaa344. PMC 7184337. PMID 32221519.
- Jiang HW, Li Y, Zhang HN, Wang W, Yang X, Qi H, et al. (July 2020). "SARS-CoV-2 proteome microarray for global profiling of COVID-19 specific IgG and IgM responses". Nature Communications (Nature Communications ed.). 11 (1): 3581. doi:10.1038/s41467-020-17488-8. PMC 7360742. PMID 32665645.
- Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, et al. (April 2010). "Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada". PLOS Medicine. 7 (4): e1000258. doi:10.1371/journal.pmed.1000258. PMC 2850386. PMID 20386731.
- Gotoff R, Tamura M, Janus J, Thompson J, Wright P, Ennis FA (January 1994). "Primary influenza A virus infection induces cross-reactive antibodies that enhance uptake of virus into Fc receptor-bearing cells". The Journal of Infectious Diseases. 169 (1): 200–3. doi:10.1093/infdis/169.1.200. PMID 8277183.
- Boonnak K, Slike BM, Burgess TH, Mason RM, Wu SJ, Sun P, et al. (April 2008). "Role of dendritic cells in antibody-dependent enhancement of dengue virus infection". Journal of Virology. 82 (8): 3939–51. doi:10.1128/JVI.02484-07. PMC 2292981. PMID 18272578.
- Ambuel Y, Young G, Brewoo JN, Paykel J, Weisgrau KL, Rakasz EG, et al. (15 September 2014). "A rapid immunization strategy with a live-attenuated tetravalent dengue vaccine elicits protective neutralizing antibody responses in non-human primates". Frontiers in Immunology. 5 (2014): 263. doi:10.3389/fimmu.2014.00263. PMC 4046319. PMID 24926294.
- Guzman MG, Vazquez S (December 2010). "The complexity of antibody-dependent enhancement of dengue virus infection". Viruses. 2 (12): 2649–62. doi:10.3390/v2122649. PMC 3185591. PMID 21994635.
- King CA, Anderson R, Marshall JS (August 2002). "Dengue virus selectively induces human mast cell chemokine production". Journal of Virology. 76 (16): 8408–19. doi:10.1128/JVI.76.16.8408-8419.2002. PMC 155122. PMID 12134044.
- Normile D (October 2013). "Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts". Science. 342 (6157): 415. Bibcode:2013Sci...342..415N. doi:10.1126/science.342.6157.415. PMID 24159024.
- Alvarez G, Piñeros JG, Tobón A, Ríos A, Maestre A, Blair S, Carmona-Fonseca J (October 2006). "Efficacy of three chloroquine-primaquine regimens for treatment of Plasmodium vivax malaria in Colombia". The American Journal of Tropical Medicine and Hygiene. 75 (4): 605–9. doi:10.4269/ajtmh.2006.75.605. PMID 17038680.
- Guzman MG, Alvarez M, Rodriguez-Roche R, Bernardo L, Montes T, Vazquez S, et al. (February 2007). "Neutralizing antibodies after infection with dengue 1 virus". Emerging Infectious Diseases. 13 (2): 282–6. doi:10.3201/eid1302.060539. PMC 2725871. PMID 17479892.
- Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ (May 2007). "Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention". Proceedings of the National Academy of Sciences of the United States of America. 104 (22): 9422–7. Bibcode:2007PNAS..104.9422G. doi:10.1073/pnas.0703498104. PMC 1868655. PMID 17517625.
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P (November 1985). "A Trojan Horse mechanism for the spread of visna virus in monocytes". Virology. 147 (1): 231–6. doi:10.1016/0042-6822(85)90246-6. PMID 2998068.
- Chen YC, Wang SY (October 2002). "Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide". Journal of Virology. 76 (19): 9877–87. doi:10.1128/JVI.76.19.9877-9887.2002. PMC 136495. PMID 12208965.
- Witayathawornwong P (January 2005). "Fatal dengue encephalitis" (PDF). The Southeast Asian Journal of Tropical Medicine and Public Health. 36 (1): 200–2. PMID 15906668. Archived from the original (PDF) on 24 July 2011.
- Rodenhuis-Zybert IA, Wilschut J, Smit JM (August 2010). "Dengue virus life cycle: viral and host factors modulating infectivity". Cellular and Molecular Life Sciences. 67 (16): 2773–86. doi:10.1007/s00018-010-0357-z. PMID 20372965. S2CID 4232236.
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. (December 2010). "Dengue: a continuing global threat". Nature Reviews. Microbiology. 8 (12 Suppl): S7-16. doi:10.1038/nrmicro2460. PMC 4333201. PMID 21079655.
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. (May 2010). "Cross-reacting antibodies enhance dengue virus infection in humans". Science. 328 (5979): 745–8. Bibcode:2010Sci...328..745D. doi:10.1126/science.1185181. PMC 3837288. PMID 20448183.
- Modhiran N, Kalayanarooj S, Ubol S (December 2010). "Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse". PLOS Neglected Tropical Diseases. PLOS ONE. 4 (12): e924. doi:10.1371/journal.pntd.0000924. PMC 3006139. PMID 21200427.
- Guzman MG (2000). "Dr. Guzman et al. Respond to Dr. Vaughn". American Journal of Epidemiology. 152 (9): 804. doi:10.1093/aje/152.9.804.
- Willey S, Aasa-Chapman MM, O'Farrell S, Pellegrino P, Williams I, Weiss RA, Neil SJ (March 2011). "Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralising antibodies at early stages of infection". Retrovirology. 8: 16. doi:10.1186/1742-4690-8-16. PMC 3065417. PMID 21401915.
- Levy JA (2007). HIV and the pathogenesis of AIDS. Wiley-Blackwell. p. 247. ISBN 978-1-55581-393-2.
- Yu Q, Yu R, Qin X (September 2010). "The good and evil of complement activation in HIV-1 infection". Cellular & Molecular Immunology. 7 (5): 334–40. doi:10.1038/cmi.2010.8. PMC 4002684. PMID 20228834.
- Gras GS, Dormont D (January 1991). "Antibody-dependent and antibody-independent complement-mediated enhancement of human immunodeficiency virus type 1 infection in a human, Epstein-Barr virus-transformed B-lymphocytic cell line". Journal of Virology. 65 (1): 541–5. doi:10.1128/JVI.65.1.541-545.1991. PMC 240554. PMID 1845908.
- Bouhlal H, Chomont N, Réquena M, Nasreddine N, Saidi H, Legoff J, et al. (January 2007). "Opsonization of HIV with complement enhances infection of dendritic cells and viral transfer to CD4 T cells in a CR3 and DC-SIGN-dependent manner". Journal of Immunology. 178 (2): 1086–95. doi:10.4049/jimmunol.178.2.1086. PMID 17202372.
- Bouhlal H, Chomont N, Haeffner-Cavaillon N, Kazatchkine MD, Belec L, Hocini H (September 2002). "Opsonization of HIV-1 by semen complement enhances infection of human epithelial cells". Journal of Immunology. 169 (6): 3301–6. doi:10.4049/jimmunol.169.6.3301. PMID 12218150.
- Subbramanian RA, Xu J, Toma E, Morisset R, Cohen EA, Menezes J, Ahmad A (June 2002). "Comparison of human immunodeficiency virus (HIV)-specific infection-enhancing and -inhibiting antibodies in AIDS patients". Journal of Clinical Microbiology. 40 (6): 2141–6. doi:10.1128/JCM.40.6.2141-2146.2002. PMC 130693. PMID 12037078.
- Beck Z, Prohászka Z, Füst G (June 2008). "Traitors of the immune system-enhancing antibodies in HIV infection: their possible implication in HIV vaccine development". Vaccine. 26 (24): 3078–85. doi:10.1016/j.vaccine.2007.12.028. PMC 7115406. PMID 18241961.
- Robinson WE, Montefiori DC, Mitchell WM (April 1990). "Complement-mediated antibody-dependent enhancement of HIV-1 infection requires CD4 and complement receptors". Virology. 175 (2): 600–4. doi:10.1016/0042-6822(90)90449-2. PMID 2327077.
- Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, et al. (March 2005). "Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial". The Journal of Infectious Diseases. 191 (5): 666–77. doi:10.1086/428405. PMID 15688279.
- Takada A, Kawaoka Y (2003). "Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications". Reviews in Medical Virology. 13 (6): 387–98. doi:10.1002/rmv.405. PMID 14625886.
- Takada A, Feldmann H, Ksiazek TG, Kawaoka Y (July 2003). "Antibody-dependent enhancement of Ebola virus infection". Journal of Virology. 77 (13): 7539–44. doi:10.1128/JVI.77.13.7539-7544.2003. PMC 164833. PMID 12805454.