Heart failure
Heart failure (HF), also known as congestive heart failure (CHF) and congestive cardiac failure (CCF), is when the heart is unable to pump sufficiently to maintain blood flow to meet the body's needs.[11][12][13] Signs and symptoms of heart failure commonly include shortness of breath, excessive tiredness, and leg swelling.[4] The shortness of breath is usually worse with exercise or while lying down, and may wake the person at night.[4] A limited ability to exercise is also a common feature.[14] Chest pain, including angina, does not typically occur due to heart failure.[15]
| Heart failure | |
|---|---|
| Other names | Chronic heart failure (CHF), congestive cardiac failure (CCF)[1][2][3] |
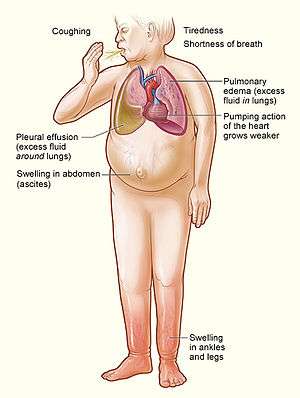 | |
| Signs and symptoms of severe heart failure | |
| Specialty | Cardiology |
| Symptoms | Shortness of breath, feeling tired, leg swelling[4] |
| Duration | Usually lifelong |
| Causes | Heart attack, high blood pressure, abnormal heart rhythm, excessive alcohol use, infection, heart damage[4][5] |
| Risk factors | Smoking, sedentary lifestyle |
| Diagnostic method | Echocardiogram[6] |
| Differential diagnosis | Kidney failure, thyroid disease, liver disease, anemia, obesity[7] |
| Medication | Diuretics, cardiac medications[6][8] |
| Frequency | 40 million (2015),[9] 1–2% of adults (developed countries)[5][10] |
| Deaths | 35% risk of death in first year[4] |
Common causes of heart failure include coronary artery disease, including a previous myocardial infarction (heart attack), high blood pressure, atrial fibrillation, valvular heart disease, excess alcohol use, infection, and cardiomyopathy of an unknown cause.[4][5] These cause heart failure by changing either the structure or the function of the heart.[4] The two types of left ventricular heart failure – heart failure with reduced ejection fraction (HFrEF), and heart failure with preserved ejection fraction (HFpEF) – are based on whether the ability of the left ventricle to contract, or to relax, is affected.[4] The severity of the heart failure is graded by the severity of symptoms with exercise.[7] Heart failure is not the same as heart attack (in which part of the heart muscle dies) or cardiac arrest (in which blood flow stops altogether).[16][17] Other diseases that may have symptoms similar to heart failure include obesity, kidney failure, liver problems, anemia, and thyroid disease.[7] Diagnosis is based on symptoms, physical findings, and echocardiography.[6] Blood tests, electrocardiography, and chest radiography may be useful to determine the underlying cause.[6]
Treatment depends on the severity and cause of the disease.[6] In people with chronic stable mild heart failure, treatment commonly consists of lifestyle modifications such as stopping smoking, physical exercise, and dietary changes, as well as medications.[8][18] In those with heart failure due to left ventricular dysfunction, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or valsartan/sacubitril along with beta blockers are recommended.[6][19] For those with severe disease, aldosterone antagonists, or hydralazine with a nitrate may be used.[6] Diuretics are useful for preventing fluid retention and the resulting shortness of breath.[8] Sometimes, depending on the cause, an implanted device such as a pacemaker or an implantable cardiac defibrillator (ICD) may be recommended.[6] In some moderate or severe cases, cardiac resynchronization therapy (CRT)[20] or cardiac contractility modulation may be of benefit.[21] A ventricular assist device (for the left, right, or both ventricles), or occasionally a heart transplant may be recommended in those with severe disease that persists despite all other measures.[8]
Heart failure is a common, costly, and potentially fatal condition.[22] In 2015, it affected about 40 million people globally.[9] Overall around 2% of adults have heart failure[22] and in those over the age of 65, this increases to 6–10%.[5][23] Rates are predicted to increase.[22] The risk of death is about 35% the first year after diagnosis, while by the second year the risk of death is less than 10% for those who remain alive.[4] This degree of risk of death is similar to some cancers.[4] In the United Kingdom, the disease is the reason for 5% of emergency hospital admissions.[4] Heart failure has been known since ancient times, with the Ebers papyrus commenting on it around 1550 BCE.[14]
Signs and symptoms
Heart failure is a pathophysiological state in which cardiac output is insufficient to meet the needs of the body and lungs.[4] The term "congestive heart failure" is often used, as one of the common symptoms is congestion, or build-up of fluid in a person's tissues and veins in the lungs or other parts of the body.[4] Specifically, congestion takes the form of water retention and swelling (edema), both as peripheral edema (causing swollen limbs and feet) and as pulmonary edema (causing breathing difficulty), as well as ascites (swollen abdomen).
Heart failure symptoms are traditionally and somewhat arbitrarily divided into "left" and "right" sided, recognizing that the left and right ventricles of the heart supply different portions of the circulation, however people commonly have both sets of signs and symptoms.
Left-sided failure
The left side of the heart receives oxygen-rich blood from the lungs and pumps it forward to the systemic circulation (the rest of the body except for the pulmonary circulation). Failure of the left side of the heart causes blood to back up (be congested) into the lungs, causing respiratory symptoms as well as fatigue due to insufficient supply of oxygenated blood. Common respiratory signs are increased rate of breathing and increased work of breathing (non-specific signs of respiratory distress). Rales or crackles, heard initially in the lung bases, and when severe, throughout the lung fields suggest the development of pulmonary edema (fluid in the alveoli). Cyanosis which suggests severe low blood oxygen, is a late sign of extremely severe pulmonary edema.
Additional signs indicating left ventricular failure include a laterally displaced apex beat (which occurs if the heart is enlarged) and a gallop rhythm (additional heart sounds) may be heard as a marker of increased blood flow or increased intra-cardiac pressure. Heart murmurs may indicate the presence of valvular heart disease, either as a cause (e.g. aortic stenosis) or as a result (e.g. mitral regurgitation) of the heart failure.
Backward failure of the left ventricle causes congestion of the lungs' blood vessels, and so the symptoms are predominantly respiratory in nature. Backward failure can be subdivided into the failure of the left atrium, the left ventricle or both within the left circuit. The person will have dyspnea (shortness of breath) on exertion and in severe cases, dyspnea at rest. Increasing breathlessness on lying flat, called orthopnea, occurs. It is often measured in the number of pillows required to lie comfortably, and in orthopnea, the person may resort to sleeping while sitting up. Another symptom of heart failure is paroxysmal nocturnal dyspnea: a sudden nighttime attack of severe breathlessness, usually several hours after going to sleep. Easy fatigability and exercise intolerance are also common complaints related to respiratory compromise.
"Cardiac asthma" or wheezing may occur.
Compromise of left ventricular forward function may result in symptoms of poor systemic circulation such as dizziness, confusion and cool extremities at rest.
Right-sided failure
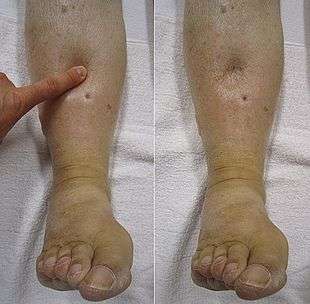
Right-sided heart failure is often caused by pulmonary heart disease (cor pulmonale), which is typically caused by difficulties of the pulmonary circulation, such as pulmonary hypertension or pulmonic stenosis.
Physical examination may reveal pitting peripheral edema, ascites, liver enlargement, and spleen enlargement. Jugular venous pressure is frequently assessed as a marker of fluid status, which can be accentuated by eliciting hepatojugular reflux. If the right ventricular pressure is increased, a parasternal heave may be present, signifying the compensatory increase in contraction strength.
Backward failure of the right ventricle leads to congestion of systemic capillaries. This generates excess fluid accumulation in the body. This causes swelling under the skin (termed peripheral edema or anasarca) and usually affects the dependent parts of the body first (causing foot and ankle swelling in people who are standing up, and sacral edema in people who are predominantly lying down). Nocturia (frequent nighttime urination) may occur when fluid from the legs is returned to the bloodstream while lying down at night. In progressively severe cases, ascites (fluid accumulation in the abdominal cavity causing swelling) and liver enlargement may develop. Significant liver congestion may result in impaired liver function (congestive hepatopathy), and jaundice and even coagulopathy (problems of decreased or increased blood clotting) may occur.
Biventricular failure
Dullness of the lung fields to finger percussion and reduced breath sounds at the bases of the lung may suggest the development of a pleural effusion (fluid collection between the lung and the chest wall). Though it can occur in isolated left- or right-sided heart failure, it is more common in biventricular failure because pleural veins drain into both the systemic and pulmonary venous systems. When unilateral, effusions are often right-sided.
If a person with a failure of one ventricle lives long enough, it will tend to progress to failure of both ventricles. For example, left ventricular failure allows pulmonary edema and pulmonary hypertension to occur, which increase stress on the right ventricle. Right ventricular failure is not as deleterious to the other side, but neither is it harmless.
Causes
Heart failure can be caused by a large number of cardiac diseases. In addition to the causes mentioned above, viral infections of the heart can lead to inflammation of the muscular layer of the heart and subsequently contribute to the development of heart failure. Genetic predisposition plays an important role. If more than one cause is present, progression is more likely and prognosis is worse.[24] Heart damage can predispose a person to develop heart failure later in life and has many causes including systemic viral infections (e.g., HIV), chemotherapeutic agents such as daunorubicin, cyclophosphamide, trastuzumab and abuse of drugs such as alcohol, cocaine, and methamphetamine. An uncommon cause is exposure to certain toxins such as lead and cobalt. Additionally, infiltrative disorders such as amyloidosis and connective tissue diseases such as systemic lupus erythematosus have similar consequences. Obstructive sleep apnea (a condition of sleep wherein disordered breathing overlaps with obesity, hypertension, and/or diabetes) is regarded as an independent cause of heart failure. Recent reports from clinical trials have also linked variation in blood pressure to heart failure[25][26] and cardiac changes that may give rise to heart failure.[27]
High output heart failure
Heart failure may also occur in situations of "high output" (termed "high-output heart failure"), where the amount of blood pumped is more than typical and the heart is unable to keep up.[28] This can occur in overload situations (blood or serum infusions), kidney diseases, chronic severe anemia, beriberi (vitamin B1/thiamine deficiency), hyperthyroidism, cirrhosis, Paget's disease, multiple myeloma, arteriovenous fistulae, or arteriovenous malformations.
Acute decompensation
Chronic stable heart failure may easily decompensate. This most commonly results from a concurrent illness (such as myocardial infarction (a heart attack) or pneumonia), abnormal heart rhythms, uncontrolled hypertension, or a person's failure to maintain a fluid restriction, diet, or medication.[29] Other factors that may worsen CHF include: anemia, hyperthyroidism, excessive fluid or salt intake, and medication such as NSAIDs and thiazolidinediones.[30] NSAIDs increase the risk twofold.[31]
Medications
A number of medications may cause or worsen the disease. This includes NSAIDS, COX-2 inhibitors, a number of anesthetic agents such as ketamine, thiazolidinediones, some cancer medications, several antiarrhythmic medications, pregabalin, alpha-2 adrenergic receptor agonists, minoxidil, itraconazole, cilostazol, anagrelide, stimulants (e.g., methylphenidate), tricyclic antidepressants, lithium, antipsychotics, dopamine agonists, TNF inhibitors, calcium channel blockers, salbutamol, and tamsulosin.[32]
By inhibiting the formation of prostaglandins, NSAIDs may exacerbate heart failure through several mechanisms including promotion of fluid retention, increasing blood pressure, and decreasing a person's response to diuretic medications.[32] Similarly, the ACC/AHA recommends against the use of COX-2 inhibitor medications in people with heart failure.[32] Thiazolidinediones have been strongly linked to new cases of heart failure and worsening of pre-existing congestive heart failure due to their association with weight gain and fluid retention.[32] Certain calcium channel blockers such as diltiazem and verapamil are known to decrease the force with which the heart ejects blood and are thus not recommended in people with heart failure with a reduced ejection fraction.[32]
Supplements
Certain alternative medicines carry a risk of exacerbating existing heart failure, and are not recommended.[32] This includes aconite, ginseng, gossypol, gynura, licorice, Lily of the valley, tetrandrine, and yohimbine.[32] Aconite can cause abnormally slow heart rates and abnormal heart rhythms such as ventricular tachycardia.[32] Ginseng can cause abnormally low or high blood pressure, and may interfere with the effects of diuretic medications.[32] Gossypol can increase the effects of diuretics, leading to toxicity.[32] Gynura can cause low blood pressure.[32] Licorice can worsen heart failure by increasing blood pressure and promoting fluid retention.[32] Lily of the valley can cause abnormally slow heart rates with mechanisms similar to those of digoxin.[32] Tetrandrine can lead to low blood pressure through inhibition of L-type calcium channels.[32] Yohimbine can exacerbate heart failure by increasing blood pressure through alpha-2 adrenergic receptor antagonism.[32]
Pathophysiology

Heart failure is caused by any condition which reduces the efficiency of the heart muscle, through damage or overloading. Over time these increases in workload, which are mediated by long-term activation of neurohormonal systems such as the renin–angiotensin system, leads to fibrosis, dilation, and structural changes in the shape of the left ventricle from elliptical to spherical.[22]
The heart of a person with heart failure may have a reduced force of contraction due to overloading of the ventricle. In a normal heart, increased filling of the ventricle results in increased contraction force by the Frank–Starling law of the heart, and thus a rise in cardiac output. In heart failure, this mechanism fails, as the ventricle is loaded with blood to the point where heart muscle contraction becomes less efficient. This is due to reduced ability to cross-link actin and myosin filaments in over-stretched heart muscle.[33]
Diagnosis
No system of diagnostic criteria has been agreed on as the gold standard for heart failure. The National Institute for Health and Care Excellence recommends measuring brain natriuretic peptide (BNP) followed by an ultrasound of the heart if positive.[34] This is recommended in those with shortness of breath.[35] In those with worsening heart failure, both a BNP and a troponin are recommended to help determine likely outcomes.[35]
Classification
One historical method of categorizing heart failure is by the side of the heart involved (left heart failure versus right heart failure). Right heart failure was thought to compromise blood flow to the lungs compared to left heart failure compromising blood flow to the aorta and consequently to the brain and the remainder of the body's systemic circulation. However, mixed presentations are common and left heart failure is a common cause of right heart failure.[36]
More accurate classification of heart failure type is made by measuring ejection fraction, or the proportion of blood pumped out of the heart during a single contraction.[37] Ejection fraction is given as a percentage with the normal range being between 50 and 75%.[37] The two types are:
1) Heart failure due to reduced ejection fraction (HFrEF). Synonyms no longer recommended are "heart failure due to left ventricular systolic dysfunction" and "systolic heart failure". HFrEF is associated with an ejection fraction of less than 40%.[38]
2) Heart failure with preserved ejection fraction (HFpEF). Synonyms no longer recommended include "diastolic heart failure" and "heart failure with normal ejection fraction."[4][18] HFpEF occurs when the left ventricle contracts normally during systole, but the ventricle is stiff and does not relax normally during diastole, which impairs filling.[4]
Heart failure may also be classified as acute or chronic. Chronic heart failure is a long-term condition, usually kept stable by the treatment of symptoms. Acute decompensated heart failure is a worsening of chronic heart failure symptoms which can result in acute respiratory distress.[39] High-output heart failure can occur when there is increased cardiac demand that results in increased left ventricular diastolic pressure which can develop into pulmonary congestion (pulmonary edema).[28]
There are several terms which are closely related to heart failure and may be the cause of heart failure, but should not be confused with it. Cardiac arrest and asystole refer to situations in which there is no cardiac output at all. Without urgent treatment, these result in sudden death. Myocardial infarction ("Heart attack") refers to heart muscle damage due to insufficient blood supply, usually as a result of a blocked coronary artery. Cardiomyopathy refers specifically to problems within the heart muscle, and these problems can result in heart failure. Ischemic cardiomyopathy implies that the cause of muscle damage is coronary artery disease. Dilated cardiomyopathy implies that the muscle damage has resulted in enlargement of the heart. Hypertrophic cardiomyopathy involves enlargement and thickening of the heart muscle.
Ultrasound
Echocardiography is commonly used to support a clinical diagnosis of heart failure. This modality uses ultrasound to determine the stroke volume (SV, the amount of blood in the heart that exits the ventricles with each beat), the end-diastolic volume (EDV, the total amount of blood at the end of diastole), and the SV in proportion to the EDV, a value known as the ejection fraction (EF). In pediatrics, the shortening fraction is the preferred measure of systolic function. Normally, the EF should be between 50% and 70%; in systolic heart failure, it drops below 40%. Echocardiography can also identify valvular heart disease and assess the state of the pericardium (the connective tissue sac surrounding the heart). Echocardiography may also aid in deciding what treatments will help the person, such as medication, insertion of an implantable cardioverter-defibrillator or cardiac resynchronization therapy. Echocardiography can also help determine if acute myocardial ischemia is the precipitating cause, and may manifest as regional wall motion abnormalities on echo.
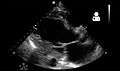 Ultrasound showing severe systolic heart failure[40]
Ultrasound showing severe systolic heart failure[40]
Chest X-ray
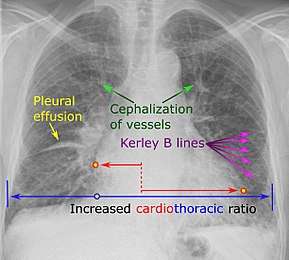
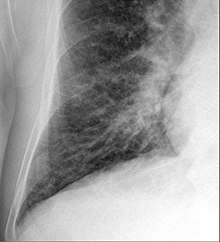
Chest X-rays are frequently used to aid in the diagnosis of CHF. In a person who is compensated, this may show cardiomegaly (visible enlargement of the heart), quantified as the cardiothoracic ratio (proportion of the heart size to the chest). In left ventricular failure, there may be evidence of vascular redistribution ("upper lobe blood diversion" or "cephalization"), Kerley lines, cuffing of the areas around the bronchi, and interstitial edema. Ultrasound of the lung may also be able to detect Kerley lines.[41]
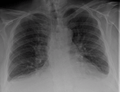 Congestive heart failure with small bilateral effusions.
Congestive heart failure with small bilateral effusions.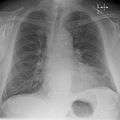 Kerley b lines.
Kerley b lines.
Electrophysiology
An electrocardiogram (ECG/EKG) may be used to identify arrhythmias, ischemic heart disease, right and left ventricular hypertrophy, and presence of conduction delay or abnormalities (e.g. left bundle branch block). Although these findings are not specific to the diagnosis of heart failure a normal ECG virtually excludes left ventricular systolic dysfunction.[42]
Blood tests
Blood tests routinely performed include electrolytes (sodium, potassium), measures of kidney function, liver function tests, thyroid function tests, a complete blood count, and often C-reactive protein if infection is suspected. An elevated brain natriuretic peptide (BNP) is a specific test indicative of heart failure. Additionally, BNP can be used to differentiate between causes of dyspnea due to heart failure from other causes of dyspnea. If myocardial infarction is suspected, various cardiac markers may be used.
BNP is a better indicator than N-terminal pro-BNP (NTproBNP) for the diagnosis of symptomatic heart failure and left ventricular systolic dysfunction. In symptomatic people, BNP had a sensitivity of 85% and specificity of 84% in detecting heart failure; performance declined with increasing age.[43]
Hyponatremia (low serum sodium concentration) is common in heart failure. Vasopressin levels are usually increased, along with renin, angiotensin II, and catecholamines in order to compensate for reduced circulating volume due to inadequate cardiac output. This leads to increased fluid and sodium retention in the body; the rate of fluid retention is higher than the rate of sodium retention in the body, this phenomenon causes "hypervolemic hyponatremia" (low sodium concentration due to high body fluid retention). This phenomenon is more common in older women with low body mass. Severe hyponatremia can result in accumulation of fluid in the brain, causing cerebral edema and intracranial hemorrhage.[44]
Angiography
Angiography is the X-ray imaging of blood vessels which is done by injecting contrast agents into the bloodstream through a thin plastic tube (catheter) which is placed directly in the blood vessel. X-ray images are called angiograms.[45] Heart failure may be the result of coronary artery disease, and its prognosis depends in part on the ability of the coronary arteries to supply blood to the myocardium (heart muscle). As a result, coronary catheterization may be used to identify possibilities for revascularisation through percutaneous coronary intervention or bypass surgery.
Algorithms
There are various algorithms for the diagnosis of heart failure. For example, the algorithm used by the Framingham Heart Study adds together criteria mainly from physical examination. In contrast, the more extensive algorithm by the European Society of Cardiology (ESC) weights the difference between supporting and opposing parameters from the medical history, physical examination, further medical tests as well as response to therapy.
Framingham criteria
By the Framingham criteria, diagnosis of congestive heart failure (heart failure with impaired pumping capability)[46] requires the simultaneous presence of at least 2 of the following major criteria or 1 major criterion in conjunction with 2 of the following minor criteria. Major criteria include an enlarged heart on a chest x-ray, an S3 gallop (a third heart sound), acute pulmonary edema, episodes of waking up from sleep gasping for air, crackles on lung auscultation, central venous pressure of more than 16 cm H
2O at the right atrium, jugular vein distension, positive abdominojugular test, and weight loss of more than 4.5 kg in 5 days in response to treatment (sometimes[47] classified as a minor criterion).[48] Minor criteria include an abnormally fast heart rate of more than 120 beats per minute, nocturnal cough, difficulty breathing with physical activity, pleural effusion, a decrease in the vital capacity by one third from maximum recorded, liver enlargement, and bilateral ankle swelling.[48]
Minor criteria are acceptable only if they can not be attributed to another medical condition such as pulmonary hypertension, chronic lung disease, cirrhosis, ascites, or the nephrotic syndrome.[48] The Framingham Heart Study criteria are 100% sensitive and 78% specific for identifying persons with definite congestive heart failure.[48]
ESC algorithm
The ESC algorithm weights the following parameters in establishing the diagnosis of heart failure:[23]
| Assessment | Diagnosis of heart failure | |
|---|---|---|
| Supports if present | Opposes if normal or absent | |
| Compatible symptoms | ++ | ++ |
| Compatible signs | ++ | + |
| Cardiac dysfunction on echocardiography | +++ | +++ |
| Response of symptoms or signs to therapy | +++ | ++ |
| ECG | ||
| Normal | ++ | |
| Abnormal | ++ | + |
| Dysrhythmia | +++ | + |
| Laboratory | ||
| Elevated BNP/NT-proBNP | +++ | + |
| Low/normal BNP/NT-proBNP | + | +++ |
| Low blood sodium | + | + |
| Kidney dysfunction | + | + |
| Mild elevations of troponin | + | + |
| Chest X-ray | ||
| Pulmonary congestion | +++ | + |
| Reduced exercise capacity | +++ | ++ |
| Abnormal pulmonary function tests | + | + |
| Abnormal hemodynamics at rest | +++ | ++ |
| + = some importance; ++ = intermediate importance; +++ = great importance. | ||
Staging
Heart failure is commonly stratified by the degree of functional impairment conferred by the severity of the heart failure (as reflected in the New York Heart Association (NYHA) Functional Classification.[49]) The NYHA functional classes (I-IV) begin with class I, which is defined as a person who experiences no limitation in any activities and has no symptoms from ordinary activities. People with NYHA class II heart failure have slight, mild limitation with everyday activities; the person is comfortable at rest or with mild exertion. With NYHA class III heart failure, there is marked limitation with any activity; the person is comfortable only at rest. A person with NYHA class IV heart failure is symptomatic at rest and becomes quite uncomfortable with any physical activity. This score documents the severity of symptoms and can be used to assess response to treatment. While its use is widespread, the NYHA score is not very reproducible and does not reliably predict the walking distance or exercise tolerance on formal testing.[50]
In its 2001 guidelines, the American College of Cardiology/American Heart Association working group introduced four stages of heart failure:[51]
- Stage A: People at high risk for developing HF in the future but no functional or structural heart disorder.
- Stage B: a structural heart disorder but no symptoms at any stage.
- Stage C: previous or current symptoms of heart failure in the context of an underlying structural heart problem, but managed with medical treatment.
- Stage D: advanced disease requiring hospital-based support, a heart transplant or palliative care.
The ACC staging system is useful since Stage A encompasses "pre-heart failure" – a stage where intervention with treatment can presumably prevent progression to overt symptoms. ACC Stage A does not have a corresponding NYHA class. ACC Stage B would correspond to NYHA Class I. ACC Stage C corresponds to NYHA Class II and III, while ACC Stage D overlaps with NYHA Class IV.
- the degree of coexisting illness: i.e. heart failure/systemic hypertension, heart failure/pulmonary hypertension, heart failure/diabetes, heart failure/kidney failure, etc.
- whether the problem is primarily increased venous back pressure (preload), or failure to supply adequate arterial perfusion (afterload).
- whether the abnormality is due to low cardiac output with high systemic vascular resistance or high cardiac output with low vascular resistance (low-output heart failure vs. high-output heart failure).
Histopathology
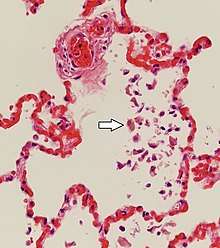
Histopathology can diagnose heart failure in autopsies. The presence of siderophages indicates chronic left-sided heart failure, but is not specific for it.[52] It is also indicated by congestion of the pulmonary circulation.
Prevention
A person's risk of developing heart failure is inversely related to their level of physical activity. Those who achieved at least 500 MET-minutes/week (the recommended minimum by U.S. guidelines) had lower heart failure risk than individuals who did not report exercising during their free time; the reduction in heart failure risk was even greater in those who engaged in higher levels of physical activity than the recommended minimum.[53] Heart failure can also be prevented by lowering high blood pressure and high blood cholesterol, and by controlling diabetes. Maintaining a healthy weight as well as decreasing sodium, alcohol, and sugar intake may help. Additionally, avoiding tobacco use has been shown to lower the risk of heart failure.[54]
Management
Treatment focuses on improving the symptoms and preventing the progression of the disease. Reversible causes of the heart failure also need to be addressed (e.g. infection, alcohol ingestion, anemia, thyrotoxicosis, arrhythmia, hypertension). Treatments include lifestyle and pharmacological modalities, and occasionally various forms of device therapy and rarely cardiac transplantation.
Acute decompensation
In acute decompensated heart failure (ADHF), the immediate goal is to re-establish adequate perfusion and oxygen delivery to end organs. This entails ensuring that airway, breathing, and circulation are adequate. Immediate treatments usually involve some combination of vasodilators such as nitroglycerin, diuretics such as furosemide, and possibly noninvasive positive pressure ventilation (NIPPV). Supplemental oxygen is indicated in those with oxygen saturation levels below 90% but is not recommended in those with normal oxygen levels on room air.[55]
Chronic management
The goals of treatment for people with chronic heart failure are the prolongation of life, the prevention of acute decompensation and the reduction of symptoms, allowing for greater activity.
Heart failure can result from a variety of conditions. In considering therapeutic options, it is important to first exclude reversible causes, including thyroid disease, anemia, chronic tachycardia, alcohol abuse, hypertension and dysfunction of one or more heart valves. Treatment of the underlying cause is usually the first approach to treating heart failure. However, in the majority of cases, either no primary cause is found or treatment of the primary cause does not restore normal heart function. In these cases, behavioral, medical and device treatment strategies exist which can provide a significant improvement in outcomes, including the relief of symptoms, exercise tolerance, and a decrease in the likelihood of hospitalization or death. Breathlessness rehabilitation for chronic obstructive pulmonary disease (COPD) and heart failure has been proposed with exercise training as a core component. Rehabilitation should also include other interventions to address shortness of breath including psychological and education needs of people and needs of carers.[56] Iron supplementation appears useful in those with iron deficiency anemia and heart failure.[57]
Monitoring
Various measures are often used to assess the progress of people being treated for heart failure. These include fluid balance (calculation of fluid intake and excretion), monitoring body weight (which in the shorter term reflects fluid shifts).[58] Remote monitoring can be effective to reduce complications for people with heart failure.[59][60]
Lifestyle
Behavior modification is a primary consideration in chronic heart failure management program, with dietary guidelines regarding fluid and salt intake.[61] Fluid restriction is important to reduce fluid retention in the body and to correct the hyponatremic status of the body.[44] The evidence of benefit of reducing salt however is poor as of 2018.[62]
Exercise should be encouraged and tailored to suit individual capabilities. The inclusion of regular physical conditioning as part of a cardiac rehabilitation program can significantly improve quality of life and reduce the risk of hospital admission for worsening symptoms; however, there is no evidence for a reduction in mortality rates as a result of exercise. Furthermore, it is not clear whether this evidence can be extended to people with heart failure with preserved ejection fraction (HFpEF) or to those whose exercise regimen takes place entirely at home.[18]
Home visits and regular monitoring at heart failure clinics reduce the need for hospitalization and improve life expectancy.[63]
Medication
First-line therapy for people with heart failure due to reduced systolic function should include angiotensin-converting enzyme (ACE) inhibitors (ACE-I) or angiotensin receptor blockers (ARBs) if the person develops a long term cough as a side effect of the ACE-I.[64] Use of medicines from this class is associated with improved survival, fewer hospitalizations for heart failure exacerbations, and improved quality of life in people with heart failure.[65]
Beta-adrenergic blocking agents (beta blockers) also form part of the first line of treatment, adding to the improvement in symptoms and mortality provided by ACE-I/ARB.[65][66] The mortality benefits of beta blockers in people with systolic dysfunction who also have atrial fibrillation (AF) is more limited than in those who do not have AF.[67] If the ejection fraction is not diminished (HFpEF), the benefits of beta blockers are more modest; a decrease in mortality has been observed but reduction in hospital admission for uncontrolled symptoms has not been observed.[68]
In people who are intolerant of ACE-I and ARBs or who have significant kidney dysfunction, the use of combined hydralazine and a long-acting nitrate, such as isosorbide dinitrate, is an effective alternate strategy. This regimen has been shown to reduce mortality in people with moderate heart failure.[69] It is especially beneficial in African-Americans (AA).[69] In AAs who are symptomatic, hydralazine and isosorbide dinitrate (H+I) can be added to ACE-I or ARBs.
In people with symptomatic heart failure with markedly reduced ejection fraction (anyone with an ejection fraction of 35% or lower or less than 40% if following a heart attack), the use of an aldosterone antagonist, in addition to beta blockers and ACE-I (once titrated to the target dose or maximum tolerated dose), can improve symptoms and reduce mortality.[70][71]
Second-line medications for CHF do not confer a mortality benefit. Digoxin is one such medication. Its narrow therapeutic window, a high degree of toxicity, and the failure of multiple trials to show a mortality benefit have reduced its role in clinical practice. It is now used in only a small number of people with refractory symptoms, who are in atrial fibrillation and/or who have chronic low blood pressure.
Diuretics have been a mainstay of treatment for treatment of fluid accumulation, and include diuretics classes such as loop diuretics, thiazide-like diuretics, and potassium-sparing diuretics. Although widely used, evidence on their efficacy and safety is limited, with the exception of mineralocorticoid antagonists such as spironolactone.[70][72] Mineralocorticoid antagonists in those under 75 years old appear to decrease the risk of death.[73] A recent Cochrane review found that in small studies, the use of diuretics appeared to have improved mortality in individuals with heart failure.[74] However, the extent to which these results can be extrapolated to a general population is unclear due to the small number of participants in the cited studies.[72]
Anemia is an independent factor in mortality in people with chronic heart failure. The treatment of anemia significantly improves quality of life for those with heart failure, often with a reduction in severity of the NYHA classification, and also improves mortality rates.[75][76] The European Society of Cardiology guideline in 2016 recommend screening for iron-deficiency anemia and treating with intravenous iron if deficiency is found.[10]
The decision to anticoagulate people with HF, typically with left ventricular ejection fractions <35% is debated, but generally, people with coexisting atrial fibrillation, a prior embolic event, or conditions which increase the risk of an embolic event such as amyloidosis, left ventricular noncompaction, familial dilated cardiomyopathy, or a thromboembolic event in a first-degree relative.[77]
Vasopressin receptor antagonists can also be used to treat heart failure. Conivaptan is the first medication approved by US Food and Drug Administration for the treatment of euvolemic hyponatremia in those with heart failure.[44] In rare cases hypertonic 3% saline together with diuretics may be used to correct hyponatremia.[44]
Sacubitril/valsartan may be used in those who still have symptoms well on an ACEI, beta blocker, and a mineralocorticoid receptor antagonist.[78] Ivabradine is recommended for people with symptomatic heart failure with reduced left ventricular ejection fraction who are receiving optimized guideline directed therapy (as above) including the maximum tolerated dose of beta blocker, have a normal heart rhythm, and continue to have a resting heart rate above 70 beats per minute.[35] Ivabradine has been found to reduce the risk of hospitalization for heart failure exacerbations in this subgroup of people with heart failure.[35]
Implanted devices
In people with severe cardiomyopathy (left ventricular ejection fraction below 35%), or in those with recurrent VT or malignant arrhythmias, treatment with an automatic implantable cardioverter defibrillator (AICD) is indicated to reduce the risk of severe life-threatening arrhythmias. The AICD does not improve symptoms or reduce the incidence of malignant arrhythmias but does reduce mortality from those arrhythmias, often in conjunction with antiarrhythmic medications. In people with left ventricular ejection (LVEF) below 35%, the incidence of ventricular tachycardia (VT) or sudden cardiac death is high enough to warrant AICD placement. Its use is therefore recommended in AHA/ACC guidelines.[20]
Cardiac contractility modulation (CCM) is a treatment for people with moderate to severe left ventricular systolic heart failure (NYHA class II–IV) which enhances both the strength of ventricular contraction and the heart's pumping capacity. The CCM mechanism is based on stimulation of the cardiac muscle by non-excitatory electrical signals (NES), which are delivered by a pacemaker-like device. CCM is particularly suitable for the treatment of heart failure with normal QRS complex duration (120 ms or less) and has been demonstrated to improve the symptoms, quality of life and exercise tolerance.[21][79][80][81][82] CCM is approved for use in Europe, but not currently in North America.[83][84]
About one third of people with LVEF below 35% have markedly altered conduction to the ventricles, resulting in dyssynchronous depolarization of the right and left ventricles. This is especially problematic in people with left bundle branch block (blockage of one of the two primary conducting fiber bundles that originate at the base of the heart and carries depolarizing impulses to the left ventricle). Using a special pacing algorithm, biventricular cardiac resynchronization therapy (CRT) can initiate a normal sequence of ventricular depolarization. In people with LVEF below 35% and prolonged QRS duration on ECG (LBBB or QRS of 150 ms or more) there is an improvement in symptoms and mortality when CRT is added to standard medical therapy.[85] However, in the two-thirds of people without prolonged QRS duration, CRT may actually be harmful.[20][21][86]
Surgical therapies
People with the most severe heart failure may be candidates for ventricular assist devices (VAD). VADs have commonly been used as a bridge to heart transplantation, but have been used more recently as a destination treatment for advanced heart failure.[87]
In select cases, heart transplantation can be considered. While this may resolve the problems associated with heart failure, the person must generally remain on an immunosuppressive regimen to prevent rejection, which has its own significant downsides.[88] A major limitation of this treatment option is the scarcity of hearts available for transplantation.
Palliative care
People with heart failure often have significant symptoms, such as shortness of breath and chest pain. Palliative care should be initiated early in the HF trajectory, and should not be an option of last resort.[89] Palliative care can not only provide symptom management, but also assist with advanced care planning, goals of care in the case of a significant decline, and making sure the person has a medical power of attorney and discussed his or her wishes with this individual.[90] A 2016 and 2017 review found that palliative care is associated with improved outcomes, such as quality of life, symptom burden, and satisfaction with care.[89][91]
Without transplantation, heart failure may not be reversible and heart function typically deteriorates with time. The growing number of people with Stage IV heart failure (intractable symptoms of fatigue, shortness of breath or chest pain at rest despite optimal medical therapy) should be considered for palliative care or hospice, according to American College of Cardiology/American Heart Association guidelines.[90]
Prognosis
Prognosis in heart failure can be assessed in multiple ways including clinical prediction rules and cardiopulmonary exercise testing. Clinical prediction rules use a composite of clinical factors such as lab tests and blood pressure to estimate prognosis. Among several clinical prediction rules for prognosticating acute heart failure, the 'EFFECT rule' slightly outperformed other rules in stratifying people and identifying those at low risk of death during hospitalization or within 30 days.[92] Easy methods for identifying people that are low-risk are:
- ADHERE Tree rule indicates that people with blood urea nitrogen < 43 mg/dl and systolic blood pressure at least 115 mm Hg have less than 10% chance of inpatient death or complications.
- BWH rule indicates that people with systolic blood pressure over 90 mm Hg, respiratory rate of 30 or fewer breaths per minute, serum sodium over 135 mmol/L, no new ST-T wave changes have less than 10% chance of inpatient death or complications.
A very important method for assessing prognosis in people with advanced heart failure is cardiopulmonary exercise testing (CPX testing). CPX testing is usually required prior to heart transplantation as an indicator of prognosis. Cardiopulmonary exercise testing involves measurement of exhaled oxygen and carbon dioxide during exercise. The peak oxygen consumption (VO2 max) is used as an indicator of prognosis. As a general rule, a VO2 max less than 12–14 cc/kg/min indicates a poor survival and suggests that the person may be a candidate for a heart transplant. People with a VO2 max<10 cc/kg/min have a clearly poorer prognosis. The most recent International Society for Heart and Lung Transplantation (ISHLT) guidelines[93] also suggest two other parameters that can be used for evaluation of prognosis in advanced heart failure, the heart failure survival score and the use of a criterion of VE/VCO2 slope > 35 from the CPX test. The heart failure survival score is a score calculated using a combination of clinical predictors and the VO2 max from the cardiopulmonary exercise test.
Heart failure is associated with significantly reduced physical and mental health, resulting in a markedly decreased quality of life.[94][95] With the exception of heart failure caused by reversible conditions, the condition usually worsens with time. Although some people survive many years, progressive disease is associated with an overall annual mortality rate of 10%.[96]
Approximately 18 of every 1000 persons will experience an ischemic stroke during the first year after diagnosis of HF. As the duration of follow-up increases, the stroke rate rises to nearly 50 strokes per 1000 cases of HF by 5 years.[97]
Epidemiology
In 2015 heart failure affected about 40 million people globally.[9] Overall around 2% of adults have heart failure[22] and in those over the age of 65, this increases to 6–10%.[5][23] Above 75 years old rates are greater than 10%.[22]
Rates are predicted to increase.[22] Increasing rates are mostly because of increasing life span, but also because of increased risk factors (hypertension, diabetes, dyslipidemia, and obesity) and improved survival rates from other types of cardiovascular disease (myocardial infarction, valvular disease, and arrhythmias).[98][99][100] Heart failure is the leading cause of hospitalization in people older than 65.[101]
United States
In the United States, heart failure affects 5.8 million people, and each year 550,000 new cases are diagnosed.[102] In 2011, heart failure was the most common reason for hospitalization for adults aged 85 years and older, and the second most common for adults aged 65–84 years.[103] It is estimated that one in five adults at age 40 will develop heart failure during their remaining lifetime and about half of people who develop heart failure die within 5 years of diagnosis.[104] Heart failure is much higher in African Americans, Hispanics, Native Americans and recent immigrants from the eastern bloc countries like Russia. This high prevalence in these ethnic minority populations has been linked to high incidence of diabetes and hypertension. In many new immigrants to the U.S., the high prevalence of heart failure has largely been attributed to lack of preventive health care or substandard treatment.[105] Nearly one out of every four people (24.7%) hospitalized in the U.S. with congestive heart failure are readmitted within 30 days.[106] Additionally, more than 50% of people seek re-admission within 6 months after treatment and the average duration of hospital stay is 6 days.
Heart failure is a leading cause of hospital readmissions in the U.S. People aged 65 and older were readmitted at a rate of 24.5 per 100 admissions in 2011. In the same year, people under Medicaid were readmitted at a rate of 30.4 per 100 admissions, and uninsured people were readmitted at a rate of 16.8 per 100 admissions. These are the highest readmission rates for both categories. Notably, heart failure was not among the top ten conditions with the most 30-day readmissions among the privately insured.[107]
United Kingdom
In the UK, despite moderate improvements in prevention, heart failure rates have increased due to population growth and ageing.[108] Overall heart failure rates are similar to the four most common causes of cancer (breast, lung, prostate and colon) combined.[108] People from deprived backgrounds are more likely to be diagnosed with heart failure and at a younger age.[108]
Developing world
In tropical countries, the most common cause of HF is valvular heart disease or some type of cardiomyopathy. As underdeveloped countries have become more affluent, there has also been an increase in the incidence of diabetes, hypertension and obesity, which have in turn raised the incidence of heart failure.[109]
Sex
Men have a higher incidence of heart failure, but the overall prevalence rate is similar in both sexes since women survive longer after the onset of heart failure.[110] Women tend to be older when diagnosed with heart failure (after menopause), they are more likely than men to have diastolic dysfunction, and seem to experience a lower overall quality of life than men after diagnosis.[110]
Economics
In 2011, non-hypertensive heart failure was one of the ten most expensive conditions seen during inpatient hospitalizations in the U.S., with aggregate inpatient hospital costs of more than $10.5 billion.[113]
Heart failure is associated with a high health expenditure, mostly because of the cost of hospitalizations; costs have been estimated to amount to 2% of the total budget of the National Health Service in the United Kingdom, and more than $35 billion in the United States.[114][115]
Research directions
There is low-quality evidence that stem cell therapy may help.[116] Although this evidence positively indicated benefit, the evidence was of lower quality than other evidence that does not indicate benefit.[117] A 2016 Cochrane review found tentative evidence of longer life expectancy and improved left ventricular ejection fraction in persons treated with bone marrow-derived stem cells.[116]
References
- "Living Well With Chronic Heart Failure" (PDF). Heart Foundation. p. 18. Archived from the original (PDF) on 22 December 2014. Retrieved 25 May 2014.
- Harrison RN, Daly L (2011). A Nurse's Survival Guide to Acute Medical Emergencies. Elsevier Health Sciences. p. 26. ISBN 978-0-7020-4900-2.
- "Congestive heart failure (CHF)". Retrieved 12 November 2018.
- National Clinical Guideline Centre (UK) (August 2010). "Chronic heart failure: National clinical guideline for diagnosis and management in primary and secondary care: Partial update". National Clinical Guideline Centre: 19–24. PMID 22741186.
- McMurray JJ, Pfeffer MA (2005). "Heart failure". Lancet. 365 (9474): 1877–89. doi:10.1016/S0140-6736(05)66621-4. PMID 15924986.
- "Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update". National Clinical Guideline Centre: 34–47. August 2010. PMID 22741186.
- "Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update". National Clinical Guideline Centre: 38–70. August 2010. PMID 22741186.
- "Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update". National Clinical Guideline Centre: 71–153. August 2010. PMID 22741186.
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. (August 2016). "2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC" (PDF). European Journal of Heart Failure (Review). 18 (8): 891–975. doi:10.1002/ejhf.592. PMID 27207191.
- "heart failure" at Dorland's Medical Dictionary
- "Heart failure". Health Information. Mayo Clinic. 23 December 2009. DS00061. Archived from the original on 13 January 2010.
- "Definition of Heart failure". Medical Dictionary. MedicineNet. 27 April 2011. Archived from the original on 8 December 2011.
- McDonagh TA (2011). Oxford textbook of heart failure. Oxford: Oxford University Press. p. 3. ISBN 978-0-19-957772-9.
- O'Connor CM (2005). Managing Acute Decompensated Heart Failure a Clinician's Guide to Diagnosis and Treatment. London: Informa Healthcare. p. 572. ISBN 978-0-203-42134-5.
- Willard & Spackman's occupational therapy. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2014. p. 1124. ISBN 978-1-4511-1080-7.
- The Cardiac Care Unit Survival Guide. Lippincott Williams & Wilkins. 2012. p. 98. ISBN 978-1-4511-7746-6.
- Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, et al. (January 2019). "Exercise-based cardiac rehabilitation for adults with heart failure". The Cochrane Database of Systematic Reviews. 1: CD003331. doi:10.1002/14651858.CD003331.pub5. PMC 6492482. PMID 30695817.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. (September 2016). "2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America". Circulation. 134 (13): e282–93. doi:10.1161/CIR.0000000000000435. PMID 27208050.
- Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA, et al. (October 2012). "2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. [corrected]". Circulation. 126 (14): 1784–800. doi:10.1161/CIR.0b013e3182618569. PMID 22965336.
- Kuck KH, Bordachar P, Borggrefe M, Boriani G, Burri H, Leyva F, et al. (January 2014). "New devices in heart failure: an European Heart Rhythm Association report: developed by the European Heart Rhythm Association; endorsed by the Heart Failure Association". Europace. 16 (1): 109–28. doi:10.1093/europace/eut311. PMID 24265466.
- Metra M, Teerlink JR (October 2017). "Heart failure". Lancet. 390 (10106): 1981–1995. doi:10.1016/S0140-6736(17)31071-1. PMID 28460827.
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. (October 2008). "ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM)". European Heart Journal. 29 (19): 2388–442. doi:10.1093/eurheartj/ehn309. PMID 18799522. Also at doi:10.1016/j.ejheart.2008.08.005
- Hazebroek MR, Moors S, Dennert R, van den Wijngaard A, Krapels I, Hoos M, et al. (September 2015). "Prognostic Relevance of Gene-Environment Interactions in Patients With Dilated Cardiomyopathy: Applying the MOGE(S) Classification". Journal of the American College of Cardiology. 66 (12): 1313–23. doi:10.1016/j.jacc.2015.07.023. PMID 26383716.
- Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. (September 2015). "Visit-to-Visit Variability of Blood Pressure and Coronary Heart Disease, Stroke, Heart Failure, and Mortality: A Cohort Study". Annals of Internal Medicine. 163 (5): 329–38. doi:10.7326/M14-2803. PMC 5021508. PMID 26215765.
- Nuyujukian DS, Koska J, Bahn G, Reaven PD, Zhou JJ (July 2020). "Blood Pressure Variability and Risk of Heart Failure in ACCORD and the VADT". Diabetes Care. 43 (7): 1471–1478. doi:10.2337/dc19-2540. PMID 32327422.
- Nwabuo CC, Yano Y, Moreira HT, Appiah D, Vasconcellos HD, Aghaji QN, et al. (April 2020). "Association Between Visit-to-Visit Blood Pressure Variability in Early Adulthood and Myocardial Structure and Function in Later Life". JAMA Cardiology. doi:10.1001/jamacardio.2020.0799. PMC 7160747. PMID 32293640.
- "high-output heart failure" at Dorland's Medical Dictionary
- Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. (April 2008). "Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF". Archives of Internal Medicine. 168 (8): 847–54. doi:10.1001/archinte.168.8.847. PMID 18443260.
- Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. (February 2005). "Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology" (PDF). European Heart Journal. 26 (4): 384–416. doi:10.1093/eurheartj/ehi044. PMID 15681577.
- Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. (August 2013). "Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials". Lancet. 382 (9894): 769–79. doi:10.1016/S0140-6736(13)60900-9. PMC 3778977. PMID 23726390.
- Page RL, O'Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, et al. (August 2016). "Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association". Circulation. 134 (6): e32–69. doi:10.1161/CIR.0000000000000426. PMID 27400984.
- Boron WF, Boulpaep EL (2005). Medical Physiology: A Cellular and Molecular Approach (Updated ed.). Saunders. p. 533. ISBN 978-0-7216-3256-8.
- Dworzynski K, Roberts E, Ludman A, Mant J (October 2014). "Diagnosing and managing acute heart failure in adults: summary of NICE guidance". BMJ. 349: g5695. doi:10.1136/bmj.g5695. PMID 25296764.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. (August 2017). "2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America". Circulation. 136 (6): e137–e161. doi:10.1161/CIR.0000000000000509. PMID 28455343.
- "Heart Failure: Signs and Symptoms". UCSF Medical Center. Archived from the original on 7 April 2014.
- "Ejection Fraction". Heart Rhythm Society. Archived from the original on 2 May 2014. Retrieved 7 June 2014.
- "Ejection Fraction Heart Failure Measurement". American Heart Association. 11 February 2014. Archived from the original on 14 July 2014. Retrieved 7 June 2014.
- Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. (April 2009). "2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation". Circulation. 119 (14): 1977–2016. doi:10.1161/CIRCULATIONAHA.109.192064. PMID 19324967.
- "UOTW #48 – Ultrasound of the Week". Ultrasound of the Week. 23 May 2015. Archived from the original on 9 May 2017. Retrieved 27 May 2017.
- Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D (August 2014). "Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis". Academic Emergency Medicine. 21 (8): 843–52. doi:10.1111/acem.12435. PMID 25176151.
- Loscalzo J, Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL (2008). Harrison's Principles of Internal Medicine (17 ed.). McGraw-Hill Medical. p. 1447. ISBN 978-0-07-147693-5.
- Ewald B, Ewald D, Thakkinstian A, Attia J (February 2008). "Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction". Internal Medicine Journal. 38 (2): 101–13. doi:10.1111/j.1445-5994.2007.01454.x. PMID 18290826.
- Abraham WT (2008). "Managing hyponatremia in heart failure". US Cardiology Review. 5 (1): 57–60. Retrieved 16 January 2018.
- "Angiography – Consumer Information – InsideRadiology". InsideRadiology. 23 September 2016. Archived from the original on 22 August 2017. Retrieved 22 August 2017.
- "congestive heart failure" at Dorland's Medical Dictionary
- Gusbi O (January 2002). "Topic Review – Heart Failure". Albany Medical Review. Archived from the original on 19 July 2012.
- "Framingham Criteria for Congestive Heart Failure". MedicalCRITERIA.com. 2005. Archived from the original on 8 October 2010. In turn citing: Framingham study 1971
- Criteria Committee, New York Heart Association (1964). Diseases of the heart and blood vessels. Nomenclature and criteria for diagnosis (6th ed.). Boston: Little, Brown. p. 114.
- Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J, Francis DP (April 2007). "Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure". Heart. 93 (4): 476–82. doi:10.1136/hrt.2006.089656. PMC 1861501. PMID 17005715.
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. (September 2005). "ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society". Circulation. 112 (12): e154–235. doi:10.1161/CIRCULATIONAHA.105.167586. PMID 16160202.
- Guido Majno; Isabelle Joris (12 August 2004). Cells, Tissues, and Disease : Principles of General Pathology. Oxford University Press. p. 620. ISBN 978-0-19-974892-1. Retrieved 19 March 2013.
- Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, et al. (November 2015). "Dose-Response Relationship Between Physical Activity and Risk of Heart Failure: A Meta-Analysis". Circulation. 132 (19): 1786–1794. doi:10.1161/CIRCULATIONAHA.115.015853. PMID 26438781.
- "Heart Failure: Am I at Risk, and Can I Prevent It?". Webmd.com. Retrieved 13 November 2018.
- "Acute heart failure with dyspnoea. First-choice treatments". Prescrire International. 27 (194): 160–162. 2018.
- Man WD, Chowdhury F, Taylor RS, Evans RA, Doherty P, Singh SJ, et al. (August 2016). "Building consensus for provision of breathlessness rehabilitation for patients with chronic obstructive pulmonary disease and chronic heart failure". Chronic Respiratory Disease. 13 (3): 229–39. doi:10.1177/1479972316642363. PMC 5029782. PMID 27072018.
- Zhou X, Xu W, Xu Y, Qian Z (August 2019). "Iron Supplementation Improves Cardiovascular Outcomes in Patients with Heart Failure". The American Journal of Medicine. 132 (8): 955–963. doi:10.1016/j.amjmed.2019.02.018. PMID 30853478.
- Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, et al. (August 2005). "Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization". Circulation. 112 (6): 841–8. doi:10.1161/CIRCULATIONAHA.104.492207. PMID 16061743.
- Bashi N, Karunanithi M, Fatehi F, Ding H, Walters D (January 2017). "Remote Monitoring of Patients With Heart Failure: An Overview of Systematic Reviews". Journal of Medical Internet Research. 19 (1): e18. doi:10.2196/jmir.6571. PMC 5291866. PMID 28108430.
- Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG (October 2015). "Structured telephone support or non-invasive telemonitoring for patients with heart failure" (PDF). The Cochrane Database of Systematic Reviews (10): CD007228. doi:10.1002/14651858.CD007228.pub3. hdl:2328/35732. PMID 26517969.
- "Lifestyle Changes for Heart Failure". American Heart Association. Archived from the original on 3 May 2015.
- Mahtani KR, Heneghan C, Onakpoya I, Tierney S, Aronson JK, Roberts N, et al. (November 2018). "Reduced Salt Intake for Heart Failure: A Systematic Review". JAMA Internal Medicine. 178 (12): 1693–1700. doi:10.1001/jamainternmed.2018.4673. PMID 30398532.
- Feltner C, Jones CD, Cené CW, Zheng ZJ, Sueta CA, Coker-Schwimmer EJ, et al. (June 2014). "Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis". Annals of Internal Medicine. 160 (11): 774–84. doi:10.7326/M14-0083. PMID 24862840.
- Goljan EF (2014). Rapid Review Pathology (4th ed.). Philadelphia, PA: Saunders/Elsevier. ISBN 978-0-323-08787-2.
- National Institute for Health and Clinical Excellence. Clinical guideline 108: Chronic heart failure – Management of chronic heart failure in adults in primary and secondary care . London, August 2010.
- Kotecha D, Manzano L, Krum H, Rosano G, Holmes J, Altman DG, et al. (April 2016). "Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis". BMJ. 353: i1855. doi:10.1136/bmj.i1855. PMC 4849174. PMID 27098105.
- Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, et al. (December 2014). "Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis" (PDF). Lancet. 384 (9961): 2235–43. doi:10.1016/S0140-6736(14)61373-8. PMID 25193873.
- Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J (5 March 2014). "Effects of beta-blockers on heart failure with preserved ejection fraction: a meta-analysis". PLOS ONE. 9 (3): e90555. Bibcode:2014PLoSO...990555L. doi:10.1371/journal.pone.0090555. PMC 3944014. PMID 24599093.
- National Clinical Guideline Centre (UK) (August 2010). "Chapter 5: Treating heart failure". Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care (Partial Update [Internet]. ed.). London (UK): Royal College of Physicians.
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. (September 1999). "The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators". The New England Journal of Medicine. 341 (10): 709–17. doi:10.1056/NEJM199909023411001. PMID 10471456.
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. (April 2003). "Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction". The New England Journal of Medicine. 348 (14): 1309–21. doi:10.1056/NEJMoa030207. PMID 12668699.
- von Lueder TG, Atar D, Krum H (October 2013). "Diuretic use in heart failure and outcomes". Clinical Pharmacology and Therapeutics. 94 (4): 490–8. doi:10.1038/clpt.2013.140. PMID 23852396.
- Japp D, Shah A, Fisken S, Denvir M, Shenkin S, Japp A (January 2017). "Mineralocorticoid receptor antagonists in elderly patients with heart failure: a systematic review and meta-analysis". Age and Ageing. 46 (1): 18–25. doi:10.1093/ageing/afw138. PMID 28181634.
- Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ (February 2012). "Diuretics for heart failure". The Cochrane Database of Systematic Reviews. 2 (2): CD003838. doi:10.1002/14651858.CD003838.pub3. PMID 22336795. (Retracted, see doi:10.1002/14651858.cd003838.pub4. If this is an intentional citation to a retracted paper, please replace
{{Retracted}}with{{Retracted|intentional=yes}}.) - He SW, Wang LX (2009). "The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review". Congestive Heart Failure. 15 (3): 123–30. doi:10.1111/j.1751-7133.2008.00030.x. PMID 19522961.
- Peraira-Moral J R, Núñez-Gil IJ (19 January 2012). "Anaemia in heart failure: intravenous iron therapy". e-Journal of the ESC Council for Cardiology Practice. 10 (16). Archived from the original on 3 June 2013. Retrieved 3 October 2012.
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. (September 2005). "ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society". Circulation. 112 (12): e154–235. doi:10.1161/CIRCULATIONAHA.105.167586. PMID 16160202.
- Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JG, et al. (October 2019). "Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology". European Journal of Heart Failure. 21 (10): 1169–1186. doi:10.1002/ejhf.1531. PMID 31129923.
- Abraham WT, Smith SA (February 2013). "Devices in the management of advanced, chronic heart failure". Nature Reviews. Cardiology. 10 (2): 98–110. doi:10.1038/nrcardio.2012.178. PMC 3753073. PMID 23229137.
- Giallauria F, Vigorito C, Piepoli MF, Stewart Coats AJ (August 2014). "Effects of cardiac contractility modulation by non-excitatory electrical stimulation on exercise capacity and quality of life: an individual patient's data meta-analysis of randomized controlled trials". International Journal of Cardiology. 175 (2): 352–7. doi:10.1016/j.ijcard.2014.06.005. PMID 24975782.
- Borggrefe M, Burkhoff D (July 2012). "Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure". European Journal of Heart Failure. 14 (7): 703–12. doi:10.1093/eurjhf/hfs078. PMID 22696514.
- Kuschyk J, Roeger S, Schneider R, Streitner F, Stach K, Rudic B, et al. (March 2015). "Efficacy and survival in patients with cardiac contractility modulation: long-term single center experience in 81 patients". International Journal of Cardiology. 183 (183C): 76–81. doi:10.1016/j.ijcard.2014.12.178. PMID 25662055.
- Kuschyk J (2014). "Der Besondere Stellenwert der Kardialen Kontraktilitätsmodulation in der Devicetherapie". Herzmedizin. Archived from the original on 5 July 2015. Retrieved 6 June 2014.
- Clinical trial number NCT01381172 for "Evaluate Safety and Efficacy of the OPTIMIZER System in Subjects With Moderate-to-Severe Heart Failure: FIX-HF-5C (FIX-HF-5C)" at ClinicalTrials.gov
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. (October 2013). "2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines". Circulation. 128 (16): e240–327. doi:10.1161/CIR.0b013e31829e8776. PMID 23741058.
- Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. (October 2013). "Cardiac-resynchronization therapy in heart failure with a narrow QRS complex" (PDF). The New England Journal of Medicine. 369 (15): 1395–405. doi:10.1056/NEJMoa1306687. PMID 23998714.
- Carrel T, Englberger L, Martinelli MV, Takala J, Boesch C, Sigurdadottir V, Gygax E, Kadner A, Mohacsi P (18 October 2012). "Continuous flow left ventricular assist devices: a valid option for heart failure patients". Swiss Medical Weekly. 142: w13701. doi:10.4414/smw.2012.13701. PMID 23135811.
- Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, et al. (December 2004). "Drug therapy in the heart transplant recipient: part I: cardiac rejection and immunosuppressive drugs". Circulation. 110 (24): 3734–40. doi:10.1161/01.cir.0000149745.83186.89. PMID 15596559.
- Kavalieratos D, Gelfman LP, Tycon LE, Riegel B, Bekelman DB, Ikejiani DZ, Goldstein N, Kimmel SE, Bakitas MA, Arnold RM (October 2017). "Palliative Care in Heart Failure: Rationale, Evidence, and Future Priorities". Journal of the American College of Cardiology. 70 (15): 1919–1930. doi:10.1016/j.jacc.2017.08.036. PMC 5731659. PMID 28982506.
- Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE (December 2009). "Palliative care in the treatment of advanced heart failure". Circulation. 120 (25): 2597–606. doi:10.1161/CIRCULATIONAHA.109.869123. PMID 20026792.
- Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. (November 2016). "Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis". JAMA. 316 (20): 2104–2114. doi:10.1001/jama.2016.16840. PMC 5226373. PMID 27893131.
- Auble TE, Hsieh M, McCausland JB, Yealy DM (August 2007). "Comparison of four clinical prediction rules for estimating risk in heart failure". Annals of Emergency Medicine. 50 (2): 127–35, 135.e1–2. doi:10.1016/j.annemergmed.2007.02.017. PMID 17449141.
- Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, et al. (September 2006). "Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006". The Journal of Heart and Lung Transplantation. 25 (9): 1024–42. doi:10.1016/j.healun.2006.06.008. PMID 16962464.
- Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M (March 2002). "Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables". Heart. 87 (3): 235–41. doi:10.1136/heart.87.3.235. PMC 1767036. PMID 11847161.
- Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK (December 2002). "Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population". European Heart Journal. 23 (23): 1867–76. doi:10.1053/euhj.2002.3255. PMID 12445536.
- Neubauer S (March 2007). "The failing heart--an engine out of fuel". The New England Journal of Medicine. 356 (11): 1140–51. doi:10.1056/NEJMra063052. PMID 17360992.
- Witt BJ, Gami AS, Ballman KV, Brown RD, Meverden RA, Jacobsen SJ, Roger VL (August 2007). "The incidence of ischemic stroke in chronic heart failure: a meta-analysis". Journal of Cardiac Failure. 13 (6): 489–96. doi:10.1016/j.cardfail.2007.01.009. PMID 17675064.
- Mann DL, Chakinala M (2012). "Chapter 234. Heart Failure and Cor Pulmonale". Harrison's principles of internal medicine: Chapter 234. Heart Failure and Cor Pulmonale (18th ed.). New York: McGraw-Hill. ISBN 978-0-07-174889-6. Archived from the original on 14 October 2013.
- Massie BM (2011). "Chapter 58: Heart Failure: Pathophysiology and Diagnosis". In Goldman L, Schafer AI (eds.). Goldman's Cecil Medicine (24th ed.). Philadelphia: Elsevier Saunders. pp. 295–302. ISBN 978-1-4377-2788-3.
- McMurray JJ, Pfeffer MA (2011). "Chapter 59: Heart Failure: Management and Diagnosis". In Goldman L, Schafer AI (eds.). Goldman's Cecil Medicine (24th ed.). Philadelphia: Elsevier Saunders. pp. 303–317. ISBN 978-1-4377-2788-3.
- Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI (January 2000). "Predictors of readmission among elderly survivors of admission with heart failure". American Heart Journal. 139 (1 Pt 1): 72–7. doi:10.1016/S0002-8703(00)90311-9. PMID 10618565.
- Bui AL, Horwich TB, Fonarow GC (January 2011). "Epidemiology and risk profile of heart failure". Nature Reviews. Cardiology. 8 (1): 30–41. doi:10.1038/nrcardio.2010.165. PMC 3033496. PMID 21060326.
- Pfuntner A, Wier LM, Stocks C (September 2013). "Most Frequent Conditions in U.S. Hospitals, 2011". HCUP Statistical Brief #162. Rockville, MD: Agency for Healthcare Research and Quality. Archived from the original on 4 March 2016. Retrieved 9 February 2016.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. (January 2013). "Heart disease and stroke statistics--2013 update: a report from the American Heart Association". Circulation. 127 (1): e6–e245. doi:10.1161/cir.0b013e31828124ad. PMC 5408511. PMID 23239837.
- "Heart Failure Information". Archived from the original on 24 January 2010. Retrieved 21 January 2010.
- Elixhauser A, Steiner C. Readmissions to U.S. Hospitals by Diagnosis, 2010. HCUP Statistical Brief #153. Agency for Healthcare Research and Quality. April 2013. "Statistical Brief #153". Archived from the original on 18 April 2015. Retrieved 8 May 2013.
- Hines AL, Barrett ML, Jiang HJ, Steiner CA (April 2014). "Conditions With the Largest Number of Adult Hospital Readmissions by Payer, 2011". HCUP Statistical Brief #172. Rockville, MD: Agency for Healthcare Research and Quality. Archived from the original on 4 March 2016.
- Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJ, Rahimi K (February 2018). "Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals". Lancet. 391 (10120): 572–580. doi:10.1016/S0140-6736(17)32520-5. PMC 5814791. PMID 29174292.
- Melmed 2011, p. 146
- Strömberg A, Mårtensson J (April 2003). "Gender differences in patients with heart failure". European Journal of Cardiovascular Nursing. 2 (1): 7–18. doi:10.1016/S1474-5151(03)00002-1. PMID 14622644.
- Solanki P (2015). "Heart Failure in South Asian Population". Management of Heart Failure. pp. 305–317. doi:10.1007/978-1-4471-6657-3_16. ISBN 978-1-4471-6656-6.
- Reyes EB, Ha JW, Firdaus I, Ghazi AM, Phrommintikul A, Sim D, et al. (November 2016). "Heart failure across Asia: Same healthcare burden but differences in organization of care". International Journal of Cardiology. 223: 163–167. doi:10.1016/j.ijcard.2016.07.256. PMID 27541646.
- Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. HCUP Statistical Brief #160. Agency for Healthcare Research and Quality, Rockville, MD. August 2013. "Statistical Brief #160". Archived from the original on 14 March 2017. Retrieved 1 May 2017.
- Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ (June 2002). "The current cost of heart failure to the National Health Service in the UK". European Journal of Heart Failure. 4 (3): 361–71. doi:10.1016/S1388-9842(01)00198-2. PMID 12034163.
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. (January 2008). "Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee". Circulation. 117 (4): e25–146. doi:10.1161/CIRCULATIONAHA.107.187998. PMID 18086926.
- Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E (December 2016). "Stem cell therapy for chronic ischaemic heart disease and congestive heart failure". The Cochrane Database of Systematic Reviews. 12: CD007888. doi:10.1002/14651858.CD007888.pub3. PMC 6463978. PMID 28012165.
- Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, Howard JP, Cole GD, Francis DP (April 2014). "Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis". BMJ. 348: g2688. doi:10.1136/bmj.g2688. PMC 4002982. PMID 24778175.
External links
| Wikimedia Commons has media related to Heart failure. |
- Heart failure, American Heart Association – information and resources for treating and living with heart failure
- Heart Failure Matters – patient information website of the Heart Failure Association of the European Society of Cardiology
- Heart failure in children by Great Ormond Street Hospital, London, UK
- "Heart Failure". MedlinePlus. U.S. National Library of Medicine.
| Classification | |
|---|---|
| External resources |