Norovirus
Norovirus, sometimes referred to as the winter vomiting bug, is the most common cause of gastroenteritis.[1][6] Infection is characterized by non-bloody diarrhea, vomiting, and stomach pain.[2][3] Fever or headaches may also occur.[2] Symptoms usually develop 12 to 48 hours after being exposed, and recovery typically occurs within 1 to 3 days.[2] Complications are uncommon, but may include dehydration, especially in the young, the old, and those with other health problems.[2]
| Norovirus | |
|---|---|
| Other names | Winter vomiting bug[1] |
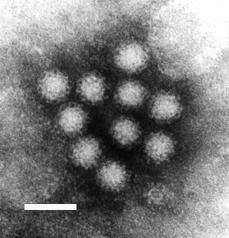 | |
| Transmission electron micrograph of Norwalk virus. The white bar = 50 nm | |
| Specialty | Emergency medicine, pediatrics |
| Symptoms | Diarrhea, vomiting, stomach pain, headache[2] |
| Complications | Dehydration[2] |
| Usual onset | 12 to 48 hours after exposure[2] |
| Duration | 1 to 3 days[2] |
| Causes | Norovirus[3] |
| Diagnostic method | Based on symptoms[3] |
| Prevention | Hand washing, disinfection of contaminated surfaces[4] |
| Treatment | Supportive care (drinking sufficient fluids or intravenous fluids)[5] |
| Frequency | 685 million cases per year[6] |
| Deaths | 200,000 per year[6][7] |
The virus is usually spread by the fecal–oral route.[3] This may be through contaminated food or water or person-to-person contact.[3] It may also spread via contaminated surfaces or through air from the vomit of an infected person.[3] Risk factors include unsanitary food preparation and sharing close quarters.[3] Diagnosis is generally based on symptoms.[3] Confirmatory testing is not usually available but may be performed during outbreaks by public health agencies.[3]
Prevention involves proper hand washing and disinfection of contaminated surfaces.[4] Alcohol-based hand sanitizers can be used in addition but are less effective than hand washing.[4] There is no vaccine or specific treatment for norovirus.[4][5] Management involves supportive care such as drinking sufficient fluids or intravenous fluids.[5] Oral rehydration solutions are the preferred fluids to drink, although other drinks without caffeine or alcohol can help.[5]
Norovirus results in about 685 million cases of disease and 200,000 deaths globally a year.[6][7] It is common both in the developed and developing world.[3][8] Those under the age of five are most often affected, and in this group it results in about 50,000 deaths in the developing world.[6] Norovirus infections occur more commonly during winter months.[6] It often occurs in outbreaks, especially among those living in close quarters.[3] In the United States, it is the cause of about half of all foodborne disease outbreaks.[3] The virus is named after the city of Norwalk, Ohio, where an outbreak occurred in 1968.[9][10]
Signs and symptoms
Norovirus infection is characterized by nausea, vomiting, watery diarrhea, abdominal pain, and in some cases, loss of taste. A person usually develops symptoms of gastroenteritis 12 to 48 hours after being exposed to norovirus.[11] General lethargy, weakness, muscle aches, headaches, and low-grade fevers may occur. The disease is usually self-limiting, and severe illness is rare. Although having norovirus can be unpleasant, it is not usually dangerous, and most who contract it make a full recovery within two to three days.[1]
Norovirus can establish a long term infection in people who are immunocompromised, such as those with common variable immunodeficiency or with a suppressed immune system after organ transplantation.[12] These infections can be with or without symptoms.[12] In severe cases, persistent infections can lead to norovirus‐associated enteropathy, intestinal villous atrophy, and malabsorption.[12]
Virology
| Norovirus | |
|---|---|
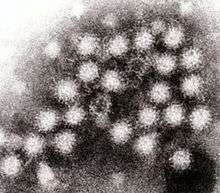 | |
| Transmission electron micrograph of Norovirus particles in feces | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Pisuviricota |
| Class: | Pisoniviricetes |
| Order: | Picornavirales |
| Family: | Caliciviridae |
| Genus: | Norovirus |
| Type species | |
| Norwalk virus | |
| Species | |
| |
Transmission
Noroviruses are transmitted directly from person to person (62–84% of all reported outbreaks)[13] and indirectly via contaminated water and food. They are extremely contagious, and fewer than twenty virus particles can cause an infection[14] (some research suggests as few as five).[15] Transmission can be aerosolized when those stricken with the illness vomit, and can be aerosolized by a toilet flush when vomit or diarrhea is present; infection can follow eating food or breathing air near an episode of vomiting, even if cleaned up.[16] The viruses continue to be shed after symptoms have subsided and shedding can still be detected many weeks after infection.[17]
Vomiting, in particular, transmits infection effectively and appears to allow airborne transmission. In one incident, a person who vomited spread infection across a restaurant, suggesting that many unexplained cases of food poisoning may have their source in vomit.[18] In December 1998, 126 people were dining at six tables; one woman vomited onto the floor. Staff quickly cleaned up, and people continued eating. Three days later others started falling ill; 52 people reported a range of symptoms, from fever and nausea to vomiting and diarrhea. The cause was not immediately identified. Researchers plotted the seating arrangement: more than 90% of the people at the same table as the sick woman later reported becoming ill. There was a direct correlation between the risk of infection of people at other tables and how close they were to the sick woman. More than 70% of the diners at an adjacent table fell ill; at a table on the other side of the restaurant, the infection rate was still 25%. The outbreak was attributed to a Norwalk-like virus (norovirus). Other cases of transmission by vomit were later identified.[19]
In one outbreak at an international scout jamboree in the Netherlands, each person with gastroenteritis infected an average of 14 people before increased hygiene measures were put in place. Even after these new measures were enacted, an ill person still infected an average of 2.1 other people.[20] A US Centers for Disease Control and Prevention (CDC) study of 11 outbreaks in New York State lists the suspected mode of transmission as person-to-person in seven outbreaks, foodborne in two, waterborne in one, and one unknown. The source of waterborne outbreaks may include water from municipal supplies, wells, recreational lakes, swimming pools, and ice machines.[21]
Shellfish and salad ingredients are the foods most often implicated in norovirus outbreaks. Ingestion of shellfish that have not been sufficiently heated – under 75 °C (167 °F) – poses a high risk for norovirus infection.[22][23] Foods other than shellfish may be contaminated by infected food handlers.[24] Many norovirus outbreaks have been traced to food that was handled by one infected person.[25]
Classification
Noroviruses (NoV) are a genetically diverse group of single-stranded positive-sense RNA, non-enveloped viruses belonging to the family Caliciviridae.[26][27] According to the International Committee on Taxonomy of Viruses, the genus Norovirus has one species, which is called Norwalk virus.[26] Serotypes, strains and isolates include:[28]
- Norwalk virus
- Hawaii virus
- Snow Mountain virus
- Mexico virus
- Desert Shield virus
- Southampton virus
- Lordsdale virus
- Wilkinson virus[29]
Noroviruses commonly isolated in cases of acute gastroenteritis belong to two genogroups: genogroup I (GI) includes Norwalk virus, Desert Shield virus and Southampton virus; and II (GII), which includes Bristol virus, Lordsdale virus, Toronto virus, Mexico virus, Hawaii virus and Snow Mountain virus.[27]
Noroviruses can genetically be classified into at least seven different genogroups (GI, GII, GIII, GIV, GV, GVI, and GVII), which can be further divided into different genetic clusters or genotypes.[30] For example, genogroup II, the most prevalent human genogroup, presently contains 19 genotypes. Genogroups I, II and IV infect humans, whereas genogroup III infects bovine species, and genogroup V has recently been isolated in mice.[29]
Most noroviruses that infect humans belong to genogroups GI and GII.[31] Noroviruses from genogroup II, genotype 4 (abbreviated as GII.4) account for the majority of adult outbreaks of gastroenteritis and often sweep across the globe.[32] Recent examples include US95/96-US strain, associated with global outbreaks in the mid- to late-1990s; Farmington Hills virus associated with outbreaks in Europe and the United States in 2002 and in 2004; and Hunter virus which was associated with outbreaks in Europe, Japan and Australasia. In 2006, there was another large increase in NoV infection around the globe.[33] Reports have shown a link between the expression of human histo-blood group antigens (HBGAs) and the susceptibility to norovirus infection. Studies have suggested the capsid of noroviruses may have evolved from selective pressure of human HBGAs.[34]
A 2008 study suggests the protein MDA-5 may be the primary immune sensor that detects the presence of noroviruses in the body.[35] Some people have common variations of the MDA-5 gene that could make them more susceptible to norovirus infection.[36]
A 2010 study suggested a specific genetic version of norovirus (which would not be distinguishable from other types of the virus using standard viral antibody tests) interacts with a specific mutation in the ATG16L1 gene to help trigger symptomatic Crohn's disease in mice that have been subjected to a chemical that causes intestinal injury similar to the process in humans. (There are other similar ways for such diseases to happen like this, and this study in itself does not prove norovirus causes Crohn's in humans).
Structure
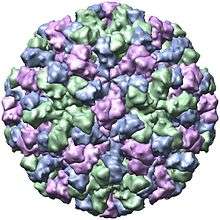
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Norovirus | Icosahedral | T=1, T=3 | Non-enveloped | Linear | Monopartite |
Viruses in Norovirus are non-enveloped, with icosahedral geometries. Capsid diameters vary widely, from 23–40 nm in diameter. The larger capsids (38–40 nm) exhibit T=3 symmetry and are composed of 180 VP1 proteins. Small capsids (23 nm) show T=1 symmetry, and are composed of 60 VP1 proteins.[37] The virus particles demonstrate an amorphous surface structure when visualized using electron microscopy.[38]
Genome
Noroviruses contain a linear, non-segmented,[37] positive-sense RNA genome of approximately 7.5 kilobases, encoding a large polyprotein which is cleaved into six smaller non-structural proteins (NS1/2 to NS7)[39] by the viral 3C-like protease (NS6), a major structural protein (VP1) of about 58~60 kDa and a minor capsid protein (VP2).[40]
The most variable region of the viral capsid is the P2 domain, which contains antigen-presenting sites and carbohydrate-receptor binding regions.[41][42][43][44][45]
Evolution
Groups 1, 2, 3, and 4 last shared a common ancestor in AD 867.[46] The group 2 and group 4 viruses last shared a common ancestor in approximately AD 1443 (95% highest posterior density 1336–1542 AD).[47] Several estimates of the evolution rate have been made varying from 8.98 × 10−3 to 2.03 × 10−3 substitutions per site per year.
The estimated mutation rate (1.21×10−2 to 1.41 ×10−2 substitutions per site per year) in this virus is high even compared with other RNA viruses.[48]
In addition, a recombination hotspot exists at the ORF1-ORF2 (VP1) junction.[49]
Replication cycle
Viral replication is cytoplasmic. Entry into the host cell is achieved by attachment to host receptors, which mediates endocytosis. Positive-stranded RNA virus transcription is the method of replication. Translation takes place by leaky scanning and RNA termination-reinitiation. Humans and other mammals serve as the natural host. Transmission routes are fecal-oral and contamination.[37]
| Genus | Host details | Tissue tropism | Entry details | Release details | Replication site | Assembly site | Transmission |
|---|---|---|---|---|---|---|---|
| Norovirus | Humans; mammals | Intestinal epithelium | Cell receptor endocytosis | Lysis | Cytoplasm | Cytoplasm | Oral-fecal |
Pathophysiology
When a person becomes infected with norovirus, the virus is replicated within the small intestine. After approximately one to two days, norovirus infection symptoms can appear. The principal symptom is acute gastroenteritis that develops between 12 and 48 hours after exposure, and lasts for 24–72 hours.[50] The disease is usually self-limiting, and characterized by nausea, forceful vomiting, watery diarrhea, and abdominal pain, and in some cases, loss of taste. General lethargy, weakness, muscle aches, headache, cough, and low-grade fever may occur.
Severe illness is rare; although people are frequently treated at the emergency ward, they are rarely admitted to the hospital. The number of deaths from norovirus in the United States is estimated to be around 570–800[51] each year, with most of these occurring in the very young, the elderly, and persons with weakened immune systems. Symptoms may become life-threatening in these groups if dehydration or electrolyte imbalance is ignored or not treated.[52]
Diagnosis
Specific diagnosis of norovirus is routinely made by polymerase chain reaction (PCR) assays or quantitative PCR assays, which give results within a few hours. These assays are very sensitive and can detect as few as 10 virus particles.[53]
Tests such as ELISA that use antibodies against a mixture of norovirus strains are available commercially, but lack specificity and sensitivity.[54]
Due to a lack of specific therapy, the need for expensive stool diagnostics is being questioned by experts if gastroenteritis by noroviruses has already been detected in the environment.[55]
Prevention
After infection, immunity to the same strain of the virus – the genotype – protects against reinfection for between 6 months to 2 years.[56] This immunity does not fully protect against infection with the other diverse genotypes of the virus.[56]
In Canada, norovirus is a notifiable disease.[57] In both the US and the UK it is not notifiable.[58][59]
Hand washing and disinfectants
Hand washing with soap and water is an effective method for reducing the transmission of norovirus pathogens. Alcohol rubs (≥62% isopropyl alcohol) may be used as an adjunct, but are less effective than hand-washing, as norovirus lacks a lipid viral envelope.[60] Surfaces where norovirus particles may be present can be sanitised with a solution of 1.5% to 7.5% of household bleach in water, or other disinfectants effective against norovirus.[50][61][62]
Health care facilities
In health-care environments, the prevention of nosocomial infections involves routine and terminal cleaning. Nonflammable alcohol vapor in CO2 systems is used in health care environments where medical electronics would be adversely affected by aerosolized chlorine or other caustic compounds.[63]
In 2011, the CDC published a clinical practice guideline addressing strategies for the prevention and control of norovirus gastroenteritis outbreaks in health-care settings.[64][65] Based on a systematic review of published scientific studies, the guideline presents 51 specific evidence-based recommendations, which were organized into 12 categories: 1) patient cohorting and isolation precautions, 2) hand hygiene, 3) patient transfer and ward closure, 4) food handlers in healthcare, 5) diagnostics, 6) personal protective equipment, 7) environmental cleaning, 8) staff leave and policy, 9) visitors, 10) education, 11) active case-finding, and 12) communication and notification. The guideline also identifies eight high-priority recommendations and suggests several areas in need of future research.
Vaccine trials
LigoCyte announced in 2007 that it was working on a vaccine and had started phase 1 trials.[66] The company has since been taken over by Takeda Pharmaceutical Company.[67] As of 2019, a bivalent (NoV GI.1/GII.4) intramuscular vaccine had completed phase 1 trials.[68][69] The vaccine relies on using a virus-like particle that is made of the norovirus capsid proteins in order to mimic the external structure of the virus. Since there is no RNA in this particle, it is incapable of reproducing and cannot cause an infection.[66]
Persistence
The norovirus can survive for long periods outside a human host depending on the surface and temperature conditions: it can survive for weeks on hard and soft surfaces,[70] and it can survive for months, maybe even years in contaminated still water.[71] A 2006 study found the virus remained on surfaces used for food preparation seven days after contamination.[72]
Detection in food
Routine protocols to detect norovirus in clams and oysters by reverse transcription polymerase chain reaction are being employed by governmental laboratories such as the Food and Drug Administration (FDA) in the US.[73]
Treatment
There is no specific medicine to treat people with norovirus illness. Norovirus infection cannot be treated with antibiotics because it is not a bacterial infection. Treatments aim to avoid complications by measures such as the management of dehydration caused by fluid loss in vomiting and diarrhea,[5] and to mitigate symptoms using antiemetics and antidiarrheals.[74]
Epidemiology
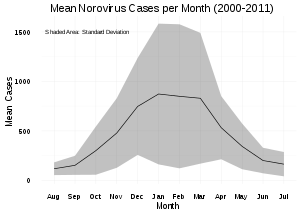
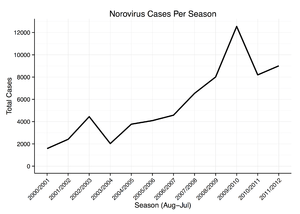
Norovirus causes about 18% of all cases of acute gastroenteritis worldwide. It is relatively common in developed countries and in low-mortality developing countries (20% and 19% respectively) compared to high-mortality developing countries (14%). Proportionately it causes more illness in people in the community or in hospital outpatients (24% and 20% respectively) as compared with hospital inpatients (17%) in whom other causes are more common.[76]
Age and emergence of new norovirus strains do not appear to affect the proportion of gastroenteritis attributable to norovirus.[76]
Norovirus is a common cause of epidemics of gastroenteritis on cruise ships. The CDC through its Vessel Sanitation Program records and investigates outbreaks of gastrointestinal illness—mostly caused by norovirus—on cruise ships with both a US and foreign itinerary;[77] there were 12 in 2015, and 10 from 1 January to 9 May 2016. An outbreak may affect over 25% of passengers, and a smaller proportion of crew members.[78]
Human genetics
Epidemiological studies have shown that individuals with different ABO(H) (histo-blood group) phenotypes are infected with NoV strains in a genotype-specific manner.[79][80] GII.4 includes global epidemic strains and binds to more histo-blood group antigens than other genogroups.[79] FUT2 fucosyl-transferase transfers a fucose sugar to the end of the ABO(H) precursor in gastrointestinal cells and saliva glands. The ABH-antigen produced is thought to act as a receptor for human norovirus: A non-functional fucosyltransferase FUT2 provides high protection from the most common norovirus strain, GII.4.[81]
Homozygous carriers of any nonsense mutation in the FUT2 gene are called non-secretors, as no ABH-antigen is produced. Approximately 20% of Caucasians are non-secretors due to G428A and C571T nonsense mutations in FUT2 and therefore have strong – although not absolute – protection from the norovirus GII.4.[82] Non-secretors can still produce ABH antigens in erythrocytes, as the precursor is formed by FUT1.[79] Some norovirus genotypes (GI.3) can infect non-secretors.[83]
History
The norovirus was originally named the "Norwalk agent" after Norwalk, Ohio, in the United States, where an outbreak of acute gastroenteritis occurred among children at Bronson Elementary School in November 1968 (although an outbreak had already been discovered in 1936 in Roskilde, Denmark, where it is commonly known as "Roskilde syge" or "Roskilde illness"). In 1972, electron microscopy on stored human stool samples identified a virus, which was given the name "Norwalk virus". Numerous outbreaks with similar symptoms have been reported since. The cloning and sequencing of the Norwalk virus genome showed that these viruses have a genomic organization consistent with viruses belonging to the family Caliciviridae.[84] The name "norovirus" (Norovirus for the genus) was approved by the International Committee on Taxonomy of Viruses (ICTV) in 2002.[85] In 2011, however, a press release and a newsletter[86] were published by ICTV, which strongly encouraged the media, national health authorities and the scientific community to use the virus name Norwalk virus, rather than the genus name Norovirus, when referring to outbreaks of the disease. This was also a public response by ICTV to the request from an individual in Japan to rename the Norovirus genus because of the possibility of negative associations for people in Japan and elsewhere who have the family name "Noro". Before this position of ICTV was made public, ICTV consulted widely with members of the Caliciviridae Study Group and carefully discussed the case.
In addition to "Norwalk agent" and "Norwalk virus", the virus has also been called "Norwalk-like virus", "small, round-structured viruses" (SRSVs), Spencer flu and "Snow Mountain virus".[87] Common names of the illness caused by noroviruses still in use include "Roskilde illness", "winter vomiting disease",[88] "winter vomiting bug",[89][90] "viral gastroenteritis", and "acute nonbacterial gastroenteritis".[52] It also colloquially is known as "stomach flu", but this actually is a broad name that refers to gastric inflammation caused by a range of viruses and bacteria.
References
- "Norovirus (vomiting bug)". nhs.uk. 2017-10-19. Retrieved 8 June 2018.
- "Norovirus Symptoms". CDC. 24 June 2016. Archived from the original on 6 December 2018. Retrieved 29 December 2017.
- Brunette GW (2017). CDC Yellow Book 2018: Health Information for International Travel. Oxford University Press. p. 269. ISBN 9780190628611.
- "Preventing Norovirus Infection". CDC. 5 May 2017. Retrieved 29 December 2017.
- "Norovirus - Treatment". CDC. Retrieved 29 December 2017.
- "Norovirus Worldwide". CDC. 15 December 2017. Archived from the original on 7 December 2018. Retrieved 29 December 2017.
- "Global Burden of Norovirus and Prospects for Vaccine Development" (PDF). CDC. August 2015. p. 3. Retrieved 29 December 2017.
- Nguyen GT, Phan K, Teng I, Pu J, Watanabe T (October 2017). "A systematic review and meta-analysis of the prevalence of norovirus in cases of gastroenteritis in developing countries". Medicine. 96 (40): e8139. doi:10.1097/MD.0000000000008139. PMC 5738000. PMID 28984764.
- Conly J, Johnston B (January 2003). "Norwalk virus - Off and running". The Canadian Journal of Infectious Diseases. 14 (1): 11–3. doi:10.1155/2003/702517. PMC 2094906. PMID 18159419.
- "Norovirus: The perfect pathogen". Knowable Magazine.
- "Norovirus | Clinical Overview | CDC". www.cdc.gov. Retrieved 2016-03-28.
- Bok, K. and Green, K. Y (2013-03-16). "Norovirus Gastroenteritis in Immunocompromised Patients". New England Journal of Medicine. 368 (10): 971. doi:10.1056/NEJMc1301022. PMC 4793940. PMID 23465122.CS1 maint: multiple names: authors list (link)
- Moore MD, Goulter RM, Jaykus L (April 2015). "Human Norovirus as a Foodborne Pathogen: Challenges and Developments". Annual Review of Food Science and Technology. 6 (1): 411–33. doi:10.1146/annurev-food-022814-015643. PMID 25884284.
- Morillo SG, Timenetsky Mdo C (2011). "Norovirus: an overview". Revista da Associação Médica Brasileira (1992). 57 (4): 453–8. doi:10.1016/s0104-4230(11)70094-x. PMID 21876931.
- Leon, Juan (2008). "Chapter 9". In Vajdy, Michael (ed.). Immunity Against Mucosal Pathogens. Springer. p. 232. ISBN 978-1-4020-8412-6.
- Robert Matthews. "I've lost my appetite..." New Scientist. Retrieved 21 February 2016.
- Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY (October 2008). "Norwalk Virus Shedding after Experimental Human Infection". Emerg. Infect. Dis. 14 (10): 1553–7. doi:10.3201/eid1410.080117. PMC 2609865. PMID 18826818.
- Marks PJ, Vipond IB, Carlisle D, Deakin D, Fey RE, Caul EO (June 2000). "Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant". Epidemiol. Infect. 124 (3): 481–487. CiteSeerX 10.1.1.404.2721. doi:10.1017/s0950268899003805. PMC 2810934. PMID 10982072.
- Marks PJ, Vipond IB, Regan FM, Wedgwood K, Fey RE, Caul EO (August 2003). "A school outbreak of Norwalk-like virus: evidence for airborne transmission". Epidemiol. Infect. 131 (1): 727–736. doi:10.1017/s0950268803008689. PMC 2870014. PMID 12948373.
- Heijne JC, Teunis P, Morroy G, Wijkmans C, Oostveen S, Duizer E, Kretzschmar M, Wallinga J (2009). "Enhanced Hygiene Measures and Norovirus Transmission during an Outbreak". Emerg. Infect. Dis. 15 (1): 24–30. doi:10.3201/eid1501.080299. PMC 2660689. PMID 19116045.
- Hedberg CW, Osterholm MT (1993). "Outbreaks of food-borne and waterborne viral gastroenteritis". Clin. Microbiol. Rev. 6 (3): 199–210. doi:10.1128/CMR.6.3.199. PMC 358282. PMID 8395330.
- "Safe Internal Cooking Temperatures Chart". Government of Canada. 7 May 2015.
- "HPA: Shellfish consumption and the risk of norovirus infection". Archived from the original on 14 July 2014. Retrieved 21 February 2016.
- Parashar UD, Monroe SS (2001). "'Norwalk-like viruses' as a cause of foodborne disease outbreaks". Rev. Med. Virol. 11 (4): 243–52. doi:10.1002/rmv.321. PMID 11479930.
- Koopmans M, Duizer E (2004). "Foodborne viruses: an emerging problem". Int. J. Food Microbiol. 90 (1): 23–41. doi:10.1016/S0168-1605(03)00169-7. PMC 7127053. PMID 14672828.
- "ICTV Report Caliciviridae".
- Department of Health and Ageing Norovirus laboratory case definition
- Schuffenecker I, Ando T, Thouvenot D, Lina B, Aymard M (2001). "Genetic classification of "Sapporo-like viruses"". Archives of Virology. 146 (11): 2115–32. doi:10.1007/s007050170024. PMID 11765915.
- Ramirez S, Giammanco GM, De Grazia S, Colomba C, Martella V, Arista S (2008). "Genotyping of GII.4 and GIIb norovirus RT-PCR amplicons by RFLP analysis". J. Virol. Methods. 147 (2): 250–6. doi:10.1016/j.jviromet.2007.09.005. PMID 17953996.
- Atmar, Robert L; Baehner, Frank; Cramer, Jakob P; Lloyd, Eric; Sherwood, James; Borkowski, Astrid; Mendelman, Paul M; Al-Ibrahim, Mohamed S; Bernstein, David L; Brandon, Donald M; Chu, Laurence; Davis, Matthew G; Epstein, Robert J; Frey, Sharon E; Rosen, Jeffrey B; Treanor, John J (15 August 2019). "Persistence of Antibodies to 2 Virus-Like Particle Norovirus Vaccine Candidate Formulations in Healthy Adults: 1-Year Follow-up With Memory Probe Vaccination". The Journal of Infectious Diseases. 220 (4): 603–614. doi:10.1093/infdis/jiz170. PMID 31001633.
- Vinjé J, Green J, Lewis DC, Gallimore CI, Brown DW, Koopmans MP (2000). "Genetic polymorphism across regions of the three open reading frames of "Norwalk-like viruses"". Arch. Virol. 145 (2): 223–41. doi:10.1007/s007050050020. PMID 10752550.
- Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI (2000). "Identification of a distinct common strain of "Norwalk-like viruses" having a global distribution". J. Infect. Dis. 179 (6): 1334–44. doi:10.1086/314783. PMID 10228052.
- Tu ET, Bull RA, Greening GE, Hewitt J, Lyon MJ, Marshall JA, McIver CJ, Rawlinson WD, White PA (2008). "Epidemics of gastroenteritis during 2006 were associated with the spread of norovirus GII.4 variants 2006a and 2006b". Clin. Infect. Dis. 46 (3): 413–20. doi:10.1086/525259. PMID 18177226.
- Shirato H (2011). "Norovirus and histo-blood group antigens". Japanese Journal of Infectious Diseases. 64 (2): 95–103. PMID 21519121.
- McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, Virgin Iv HW, Colonna M (July 18, 2008). Baric RS (ed.). "MDA-5 Recognition of a Murine Norovirus". PLOS Pathog. 4 (7): e1000108. doi:10.1371/journal.ppat.1000108. PMC 2443291. PMID 18636103.
- Researchers Discover Primary Sensor That Detects Stomach Viruses Newswise, Retrieved on July 20, 2008.
- "Viral Zone". ExPASy. Retrieved 15 June 2015.
- Prasad BV, Crawford S, Lawton JA, Pesavento J, Hardy M, Estes MK (2001). Structural studies on gastroenteritis viruses. Novartis Found. Symp. Novartis Foundation Symposia. 238. pp. 26–37, discussion 37–46. doi:10.1002/0470846534.ch3. ISBN 978-0-470-84653-7. PMID 11444031.
- Thorne LG, Goodfellow IG (February 2014). "Norovirus gene expression and replication". The Journal of General Virology. 95 (Pt 2): 278–91. doi:10.1099/vir.0.059634-0. PMID 24243731.
- Clarke IN, Lambden PR (May 2000). "Organization and expression of calicivirus genes". The Journal of Infectious Diseases. 181 Suppl 2: S309-16. doi:10.1086/315575. PMID 10804143.
- Tan M, Hegde RS, Jiang X (2004). "The P Domain of Norovirus Capsid Protein Forms Dimer and Binds to Histo-Blood Group Antigen Receptors". J. Virol. 78 (12): 6233–42. doi:10.1128/JVI.78.12.6233-6242.2004. PMC 416535. PMID 15163716.
- Tan M, Huang P, Meller J, Zhong W, Farkas T, Jiang X (2003). "Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket". J. Virol. 77 (23): 12562–71. doi:10.1128/jvi.77.23.12562-12571.2003. PMC 262557. PMID 14610179. Tan M (2004). "Erratum". J. Virol. 78 (6): 3200. CiteSeerX 10.1.1.212.5257. doi:10.1128/JVI.78.6.3201.2004.
- Cao S, Lou Z, Tan M, Chen Y, Liu Y, Zhang Z, Zhang XC, Jiang X, Li X, Rao Z (2007). "Structural Basis for the Recognition of Blood Group Trisaccharides by Norovirus". J. Virol. 81 (11): 5949–57. doi:10.1128/JVI.00219-07. PMC 1900264. PMID 17392366.
- Lundborg M, Ali E, Widmalm G (2013). "An in silico virtual screening study for the design of norovirus inhibitors: fragment-based molecular docking and binding free energy calculations". Carbohydr. Res. 378: 133–8. doi:10.1016/j.carres.2013.03.012. PMID 23582100.
- Ali ES, Rajapaksha H, Jillian MC, Petrovsky N (2016). "Norovirus drug candidates that inhibit viral capsid attachment to human histo-blood group antigens". Antiviral Res. 133: 14–22. doi:10.1016/j.antiviral.2016.07.006. PMC 5026924. PMID 27421712.
- Kobayashi M, Matsushima, Y, Motoya T, Sakon N, Shigemoto N, Okamoto-Nakagawa R et al. (2016) Molecular ecolution of the capsid gene in human norovirus genogroup II. Sci Rep 6:29400
- Ozaki K, Matsushima Y, Nagasawa K, Motoya T, Ryo A, Kuroda M, Katayama K, Kimura H (2018) Molecular evolutionary analyses of the RNA-dependent RNA polymerase region in Norovirus genogroup II Front Microbiol
- Victoria M, Miagostovich MP, Ferreira MS, Vieira CB, Fioretti JM, Leite JP, Colina R, Cristina J (2009). "Bayesian coalescent inference reveals high evolutionary rates and expansion of Norovirus populations". Infect Genet Evol. 9 (5): 927–932. doi:10.1016/j.meegid.2009.06.014. PMID 19559104.
- Tsimpidis M, Bachoumis G, Mimouli K, Kyriakopoulou Z, Robertson DL, Markoulatos P, Amoutzias GD (January 2017). "T-RECs: rapid and large-scale detection of recombination events among different evolutionary lineages of viral genomes". BMC Bioinformatics. 18 (1): 13. doi:10.1186/s12859-016-1420-z. PMC 5216575. PMID 28056784.
- "Norovirus: Technical Fact Sheet". National Center for Infectious Diseases, CDC. Archived from the original on 2012-03-08.
- Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, Parashar UD (August 2013). "Norovirus disease in the United States". Emerging Infectious Diseases. 19 (8): 1198–205. doi:10.3201/eid1908.130465. PMC 3739528. PMID 23876403.
- Goodgame R (October 2006). "Norovirus gastroenteritis". Current Gastroenterology Reports. 8 (5): 401–8. doi:10.1007/s11894-006-0026-4. PMID 16968608.
- Marshall JA, Bruggink LD (2006). "Laboratory diagnosis of norovirus". Clin. Lab. 52 (11–12): 571–81. PMID 17175887.
- Wilhelmi de Cal I, Revilla A, del Alamo JM, Román E, Moreno S, Sánchez-Fauquier A (2007). "Evaluation of two commercial enzyme immunoassays for the detection of norovirus in faecal samples from hospitalised children with sporadic acute gastroenteritis". Clin. Microbiol. Infect. 13 (3): 341–3. doi:10.1111/j.1469-0691.2006.01594.x. PMID 17391396.
- Fisman DN, Greer AL, Brouhanski G, Drews SJ (March 2009). "Of gastro and the gold standard: evaluation and policy implications of norovirus test performance for outbreak detection". Journal of Translational Medicine. 7: 23. doi:10.1186/1479-5876-7-23. PMC 2667494. PMID 19323808.
- Payne DC, Parashar UD, Lopman BA (February 2015). "Developments in understanding acquired immunity and innate susceptibility to norovirus and rotavirus gastroenteritis in children". Current Opinion in Pediatrics. 27 (1): 105–9. doi:10.1097/MOP.0000000000000166. PMC 4618547. PMID 25490691.
- "Diseases Under National Surveillance (as of January 2009)". Public Health Agency of Canada. 2003-09-17. Retrieved 21 November 2017.
- Anonymous (1 May 2010). "Notifiable diseases and causative organisms: how to report - GOV.UK". www.gov.uk. Public Health England. Retrieved 26 November 2017.
- Anonymous (28 December 2016). "Norovirus | Reporting and Surveillance | CDC". www.cdc.gov. Centers for Disease Control and Prevention. Retrieved 26 November 2017.
- Jimenez L, Chiang M (2006). "Virucidal activity of a quaternary ammonium compound disinfectant against feline calicivirus: a surrogate for norovirus". Am J Infect Control. 34 (5): 269–73. doi:10.1016/j.ajic.2005.11.009. PMID 16765204.
- "List G: EPA Registered Hospital Disinfectants Effective Against Norovirus (Norwalk-like virus)". US Environmental Protection Agency. 2015-09-28. Retrieved 9 May 2016.
- "Gastroenteritis and Noroviruses—Dr Jim Grey, Health Protection Agency". The Naked Scientists. 2007-12-09. Retrieved 2014-02-09.
- Chadwick PR, Beards G, Brown D, Caul EO, Cheesbrough J, Clarke I, Curry A, O'Brien S, Quigley K, Sellwood J, Westmoreland D (2000). "Management of hospital outbreaks of gastroenteritis due to small roundstructured viruses". J. Hosp. Infect. 45 (1): 1–10. doi:10.1053/jhin.2000.0662. PMID 10833336.
- HICPAC. "Guideline for the Prevention and Control of Norovirus Gastroenteritis Outbreaks in Healthcare Settings, 2011". Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention (CDC). Retrieved 19 March 2015.
- MacCannell T, Umscheid CA, Agarwal RK, Lee I, Kuntz G, Stevenson KB (October 2011). "Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings". Infection Control and Hospital Epidemiology. 32 (10): 939–69. doi:10.1086/662025. PMID 21931246.
- "Norovirus Vaccine" (PDF).
- "Takeda to Acquire LigoCyte Pharmaceuticals, Inc".
- Baehner, F.; Bogaerts, H.; Goodwin, R. (2016-12-01). "Vaccines against norovirus: state of the art trials in children and adults". Clinical Microbiology and Infection. Vaccines for Mutual Protection: Selected Proceedings from the 3rd ESCMID Conference on Vaccines. 22: S136–S139. doi:10.1016/j.cmi.2015.12.023. ISSN 1198-743X. PMID 27130672.
- "Key Products and Pipeline (FY2019 Q2 Pipeline Table)" (PDF). Takeda Pharmaceutical Company. 2019. Retrieved 9 December 2019.
- "How To Stay Well (When Everyone Else Is Sick)". Webmd.com. Archived from the original on 2014-01-03. Retrieved 2017-01-28.
- Frazer J (January 17, 2012). "Misery-inducing Norovirus Can Survive for Months—Perhaps Years—in Drinking Water". Scientific American. Retrieved February 27, 2012.
- D'Souza DH, Sair A, Williams K, Papafragkou E, Jean J, Moore C, Jaykus L (2006). "Persistence of caliciviruses on environmental surfaces and their transfer to food". International Journal of Food Microbiology. 108 (1): 84–91. doi:10.1016/j.ijfoodmicro.2005.10.024. PMID 16473426.
- Shieh Y, Monroe SS, Fankhauser RL, Langlois GW, Burkhardt W, Baric RS (2000). "Detection of norwalk-like virus in shellfish implicated in illness". J. Infect. Dis. 181 (Suppl 2): S360–6. doi:10.1086/315578. PMID 10804149.
- "Traveler's Diarrhea". Merck Manuals Consumer Version. Retrieved 21 February 2016.
- Smith R (24 November 2012). "Winter vomiting bug cases up 40 per cent: Health Protection Agency". Telegraph.co.uk. Retrieved 21 February 2016.
- Ahmed SM, Hall AJ, Robinson AE, et al. (August 2014). "Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis". Lancet Infect Dis. 14 (8): 725–30. doi:10.1016/S1473-3099(14)70767-4. PMID 24981041.
- CDC VSP. "Vessel Sanitation Program - Outbreak Updates for International Cruise Ships". Centers for Disease Control and Prevention. Retrieved 9 May 2016.
- "CDC - Vessel Sanitation Program - Balmoral, April 16, 2016". Cdc.gov. Retrieved 9 May 2016.
- Shirato H (2011). "Norovirus and histo-blood group antigens". Jpn. J. Infect. Dis. 64 (2): 95–103. PMID 21519121.
- Le Guyader FS, Krol J, Ambert-Balay K, Ruvoen-Clouet N, Desaubliaux B, Parnaudeau S, Le Saux JC, Ponge A, Pothier P, Atmar RL, Le Pendu J (March 2010). "Comprehensive analysis of a norovirus-associated gastroenteritis outbreak, from the environment to the consumer". Journal of Clinical Microbiology. 48 (3): 915–920. doi:10.1128/JCM.01664-09. PMC 2832421. PMID 20053852.
- Carlsson B, Kindberg E, Buesa J, Rydell GE, Lidón MF, Montava R, Abu Mallouh R, Grahn A, Rodríguez-Díaz J, Bellido J, Arnedo A, Larson G, Svensson L (May 2009). "The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 norovirus infection". PLOS ONE. 4 (5): e5593. Bibcode:2009PLoSO...4.5593C. doi:10.1371/journal.pone.0005593. PMC 2680586. PMID 19440360.
- Rydell GE, Kindberg E, Larson G, Svensson L (November 2011). "Susceptibility to winter vomiting disease: A sweet matter". Rev. Med. Virol. 21 (6): 370–382. doi:10.1002/rmv.704. PMID 22025362.
- Nordgren J, Kindberg E, Lindgren PE, Matussek A, Svensson L (January 2010). "Norovirus gastroenteritis outbreak with a secretor-independent susceptibility pattern, Sweden". Emerg. Infect. Dis. 16 (1): 81–87. doi:10.3201/eid1601.090633. PMC 2874438. PMID 20031047.
- Kapikian AZ (1996). "Overview of viral gastroenteritis". Arch. Virol. Suppl. 12: 7–19. doi:10.1007/978-3-7091-6553-9_2. ISBN 978-3-211-82875-5. PMID 9015097.
- ICTVdB Management (2006). 00.012.0.03. Norovirus. In: ICTVdB—The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed), Columbia University, New York, USA
- "2011 ICTV Newsletter #9, November 2011". ICTV. November 14, 2011.
- Appleton H (1987). "Small round viruses: classification and role in food-borne infections ...". Ciba Found. Symp. Novartis Foundation Symposia. 128: 108–25. doi:10.1002/9780470513460.ch7. ISBN 9780470513460. PMID 3036438.
- Parashar U, Quiroz ES, Mounts AW, Monroe SS, Fankhauser RL, Ando T, Noel JS, Bulens SN, Beard SR, Li JF, Bresee JS, Glass RI (2001). ""Norwalk-Like Viruses". Public Health Consequences and Outbreak Management". Morbidity and Mortality Weekly Reports—Recommendations and Reports. 50 (RR-9): 1–18. PMID 15580799.
- "Norovirus shuts wards and unit at three Sussex hospitals". BBC News. January 11, 2012. Retrieved January 20, 2012.
- "Norovirus at Norfolk hospitals: Disruption continues". BBC News. January 12, 2012. Retrieved January 20, 2012.
External links
- Norovirus (vomiting bug) NHS Norovirus infections
- Global network and database noroviruses
- CDC Viral Gastroenteritis FAQs: Center for Disease Control and Prevention of Food Illness Fact Sheet
- "Norovirus in Healthcare Facilities Fact Sheet", CDC, released December 21, 2006
- tips from CDC for cruise vacationers
- Virus Pathogen Database and Analysis Resource (ViPR): Caliciviridae
- 3D macromolecular structures of Noroviruses from the EM Data Bank(EMDB)
- Viralzone: Norovirus
- ICTV Report: Caliciviridae