Paleontology
Paleontology, also spelled palaeontology or palæontology (/ˌpeɪliɒnˈtɒlədʒi, ˌpæli-, -ən-/), is the scientific study of life that existed prior to, and sometimes including, the start of the Holocene Epoch (roughly 11,700 years before present). It includes the study of fossils to classify organisms and study interactions with each other and their environments (their paleoecology). Paleontological observations have been documented as far back as the 5th century BCE. The science became established in the 18th century as a result of Georges Cuvier's work on comparative anatomy, and developed rapidly in the 19th century. The term itself originates from Greek παλαιός, palaios, "old, ancient", ὄν, on (gen. ontos), "being, creature", and λόγος, logos, "speech, thought, study".[1]
| Part of a series on |
| Paleontology |
|---|
 |
|
Fossils
|
|
Natural history |
|
Organs and processes
|
|
Evolution
|
|
History of paleontology |
|
Branches of paleontology |
|
Paleontology Portal Category |

Paleontology lies on the border between biology and geology, but differs from archaeology in that it excludes the study of anatomically modern humans. It now uses techniques drawn from a wide range of sciences, including biochemistry, mathematics, and engineering. Use of all these techniques has enabled paleontologists to discover much of the evolutionary history of life, almost all the way back to when Earth became capable of supporting life, about 3.8 billion years ago. As knowledge has increased, paleontology has developed specialised sub-divisions, some of which focus on different types of fossil organisms while others study ecology and environmental history, such as ancient climates.
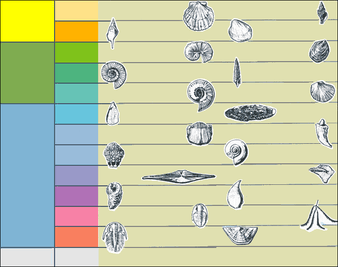
Body fossils and trace fossils are the principal types of evidence about ancient life, and geochemical evidence has helped to decipher the evolution of life before there were organisms large enough to leave body fossils. Estimating the dates of these remains is essential but difficult: sometimes adjacent rock layers allow radiometric dating, which provides absolute dates that are accurate to within 0.5%, but more often paleontologists have to rely on relative dating by solving the "jigsaw puzzles" of biostratigraphy (arrangement of rock layers from youngest to oldest). Classifying ancient organisms is also difficult, as many do not fit well into the Linnaean taxonomy classifying living organisms, and paleontologists more often use cladistics to draw up evolutionary "family trees". The final quarter of the 20th century saw the development of molecular phylogenetics, which investigates how closely organisms are related by measuring the similarity of the DNA in their genomes. Molecular phylogenetics has also been used to estimate the dates when species diverged, but there is controversy about the reliability of the molecular clock on which such estimates depend.
Overview
The simplest definition of "paleontology" is "the study of ancient life".[2] The field seeks information about several aspects of past organisms: "their identity and origin, their environment and evolution, and what they can tell us about the Earth's organic and inorganic past".[3]
A historical science
William Whewell (1794-1866) classified paleontology as one of the historical sciences, along with archaeology, geology, astronomy, cosmology, philology and history itself:[4] paleontology aims to describe phenomena of the past and to reconstruct their causes.[5] Hence it has three main elements: description of past phenomena; developing a general theory about the causes of various types of change; and applying those theories to specific facts.[6] When trying to explain the past, paleontologists and other historical scientists often construct a set of one or more hypotheses about the causes and then look for a "smoking gun", a piece of evidence that strongly accords with one hypothesis over any others.[7] Sometimes researchers discover a "smoking gun" by a fortunate accident during other research. For example, the 1980 discovery by Luis and Walter Alvarez of iridium, a mainly extraterrestrial metal, in the Cretaceous–Tertiary boundary layer made asteroid impact the most favored explanation for the Cretaceous–Paleogene extinction event - although debate continues about the contribution of volcanism.[5]
A complementary approach to developing scientific knowledge, experimental science,[8] is often said to work by conducting experiments to disprove hypotheses about the workings and causes of natural phenomena. This approach cannot prove a hypothesis, since some later experiment may disprove it, but the accumulation of failures to disprove is often compelling evidence in favor. However, when confronted with totally unexpected phenomena, such as the first evidence for invisible radiation, experimental scientists often use the same approach as historical scientists: construct a set of hypotheses about the causes and then look for a "smoking gun".[5]
Related sciences
Paleontology lies between biology and geology since it focuses on the record of past life, but its main source of evidence is fossils in rocks.[9][10] For historical reasons, paleontology is part of the geology department at many universities: in the 19th and early 20th centuries, geology departments found fossil evidence important for dating rocks, while biology departments showed little interest.[11]
Paleontology also has some overlap with archaeology, which primarily works with objects made by humans and with human remains, while paleontologists are interested in the characteristics and evolution of humans as a species. When dealing with evidence about humans, archaeologists and paleontologists may work together – for example paleontologists might identify animal or plant fossils around an archaeological site, to discover what the people who lived there ate; or they might analyze the climate at the time of habitation.[12]
In addition, paleontology often borrows techniques from other sciences, including biology, osteology, ecology, chemistry, physics and mathematics.[2] For example, geochemical signatures from rocks may help to discover when life first arose on Earth,[13] and analyses of carbon isotope ratios may help to identify climate changes and even to explain major transitions such as the Permian–Triassic extinction event.[14] A relatively recent discipline, molecular phylogenetics, compares the DNA and RNA of modern organisms to re-construct the "family trees" of their evolutionary ancestors. It has also been used to estimate the dates of important evolutionary developments, although this approach is controversial because of doubts about the reliability of the "molecular clock".[15] Techniques from engineering have been used to analyse how the bodies of ancient organisms might have worked, for example the running speed and bite strength of Tyrannosaurus,[16][17] or the flight mechanics of Microraptor.[18] It is relatively commonplace to study the internal details of fossils using X-ray microtomography.[19][20] Paleontology, biology, archaeology, and paleoneurobiology combine to study endocranial casts (endocasts) of species related to humans to clarify the evolution of the human brain.[21]
Paleontology even contributes to astrobiology, the investigation of possible life on other planets, by developing models of how life may have arisen and by providing techniques for detecting evidence of life.[22]
Subdivisions
As knowledge has increased, paleontology has developed specialised subdivisions.[23] Vertebrate paleontology concentrates on fossils from the earliest fish to the immediate ancestors of modern mammals. Invertebrate paleontology deals with fossils such as molluscs, arthropods, annelid worms and echinoderms. Paleobotany studies fossil plants, algae, and fungi. Palynology, the study of pollen and spores produced by land plants and protists, straddles paleontology and botany, as it deals with both living and fossil organisms. Micropaleontology deals with microscopic fossil organisms of all kinds.[24]

Instead of focusing on individual organisms, paleoecology examines the interactions between different ancient organisms, such as their food chains, and the two-way interactions with their environments.[25] For example, the development of oxygenic photosynthesis by bacteria caused the oxygenation of the atmosphere and hugely increased the productivity and diversity of ecosystems.[26] Together, these led to the evolution of complex eukaryotic cells, from which all multicellular organisms are built.[27]
Paleoclimatology, although sometimes treated as part of paleoecology,[24] focuses more on the history of Earth's climate and the mechanisms that have changed it[28] – which have sometimes included evolutionary developments, for example the rapid expansion of land plants in the Devonian period removed more carbon dioxide from the atmosphere, reducing the greenhouse effect and thus helping to cause an ice age in the Carboniferous period.[29]
Biostratigraphy, the use of fossils to work out the chronological order in which rocks were formed, is useful to both paleontologists and geologists.[30] Biogeography studies the spatial distribution of organisms, and is also linked to geology, which explains how Earth's geography has changed over time.[31]
Sources of evidence
Body fossils
.png)
Fossils of organisms' bodies are usually the most informative type of evidence. The most common types are wood, bones, and shells.[32] Fossilisation is a rare event, and most fossils are destroyed by erosion or metamorphism before they can be observed. Hence the fossil record is very incomplete, increasingly so further back in time. Despite this, it is often adequate to illustrate the broader patterns of life's history.[33] There are also biases in the fossil record: different environments are more favorable to the preservation of different types of organism or parts of organisms.[34] Further, only the parts of organisms that were already mineralised are usually preserved, such as the shells of molluscs. Since most animal species are soft-bodied, they decay before they can become fossilised. As a result, although there are 30-plus phyla of living animals, two-thirds have never been found as fossils.[35]
Occasionally, unusual environments may preserve soft tissues. These lagerstätten allow paleontologists to examine the internal anatomy of animals that in other sediments are represented only by shells, spines, claws, etc. – if they are preserved at all. However, even lagerstätten present an incomplete picture of life at the time. The majority of organisms living at the time are probably not represented because lagerstätten are restricted to a narrow range of environments, e.g. where soft-bodied organisms can be preserved very quickly by events such as mudslides; and the exceptional events that cause quick burial make it difficult to study the normal environments of the animals.[36] The sparseness of the fossil record means that organisms are expected to exist long before and after they are found in the fossil record – this is known as the Signor–Lipps effect.[37]
Trace fossils


Trace fossils consist mainly of tracks and burrows, but also include coprolites (fossil feces) and marks left by feeding.[32][38] Trace fossils are particularly significant because they represent a data source that is not limited to animals with easily fossilised hard parts, and they reflect organisms' behaviours. Also many traces date from significantly earlier than the body fossils of animals that are thought to have been capable of making them.[39] Whilst exact assignment of trace fossils to their makers is generally impossible, traces may for example provide the earliest physical evidence of the appearance of moderately complex animals (comparable to earthworms).[38]
Geochemical observations
Geochemical observations may help to deduce the global level of biological activity at a certain period, or the affinity of certain fossils. For example, geochemical features of rocks may reveal when life first arose on Earth,[13] and may provide evidence of the presence of eukaryotic cells, the type from which all multicellular organisms are built.[40] Analyses of carbon isotope ratios may help to explain major transitions such as the Permian–Triassic extinction event.[14]
Classifying ancient organisms
Naming groups of organisms in a way that is clear and widely agreed is important, as some disputes in paleontology have been based just on misunderstandings over names.[41] Linnaean taxonomy is commonly used for classifying living organisms, but runs into difficulties when dealing with newly discovered organisms that are significantly different from known ones. For example: it is hard to decide at what level to place a new higher-level grouping, e.g. genus or family or order; this is important since the Linnaean rules for naming groups are tied to their levels, and hence if a group is moved to a different level it must be renamed.[42]
| Tetrapods |
| ||||||||||||||||||||||||||||||||||||||||||
Warm-bloodedness evolved somewhere in the
synapsid–mammal transition.
? Warm-bloodedness must also have evolved at one of
these points – an example of convergent evolution.[43]
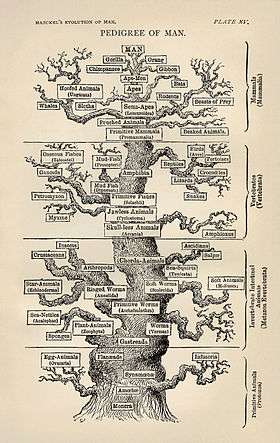
Paleontologists generally use approaches based on cladistics, a technique for working out the evolutionary "family tree" of a set of organisms.[41] It works by the logic that, if groups B and C have more similarities to each other than either has to group A, then B and C are more closely related to each other than either is to A. Characters that are compared may be anatomical, such as the presence of a notochord, or molecular, by comparing sequences of DNA or proteins. The result of a successful analysis is a hierarchy of clades – groups that share a common ancestor. Ideally the "family tree" has only two branches leading from each node ("junction"), but sometimes there is too little information to achieve this and paleontologists have to make do with junctions that have several branches. The cladistic technique is sometimes fallible, as some features, such as wings or camera eyes, evolved more than once, convergently – this must be taken into account in analyses.[43]
Evolutionary developmental biology, commonly abbreviated to "Evo Devo", also helps paleontologists to produce "family trees", and understand fossils.[44] For example, the embryological development of some modern brachiopods suggests that brachiopods may be descendants of the halkieriids, which became extinct in the Cambrian period.[45]
Estimating the dates of organisms
Paleontology seeks to map out how living things have changed through time. A substantial hurdle to this aim is the difficulty of working out how old fossils are. Beds that preserve fossils typically lack the radioactive elements needed for radiometric dating. This technique is our only means of giving rocks greater than about 50 million years old an absolute age, and can be accurate to within 0.5% or better.[46] Although radiometric dating requires very careful laboratory work, its basic principle is simple: the rates at which various radioactive elements decay are known, and so the ratio of the radioactive element to the element into which it decays shows how long ago the radioactive element was incorporated into the rock. Radioactive elements are common only in rocks with a volcanic origin, and so the only fossil-bearing rocks that can be dated radiometrically are a few volcanic ash layers.[46]
Consequently, paleontologists must usually rely on stratigraphy to date fossils. Stratigraphy is the science of deciphering the "layer-cake" that is the sedimentary record, and has been compared to a jigsaw puzzle.[47] Rocks normally form relatively horizontal layers, with each layer younger than the one underneath it. If a fossil is found between two layers whose ages are known, the fossil's age must lie between the two known ages.[48] Because rock sequences are not continuous, but may be broken up by faults or periods of erosion, it is very difficult to match up rock beds that are not directly next to one another. However, fossils of species that survived for a relatively short time can be used to link up isolated rocks: this technique is called biostratigraphy. For instance, the conodont Eoplacognathus pseudoplanus has a short range in the Middle Ordovician period.[49] If rocks of unknown age are found to have traces of E. pseudoplanus, they must have a mid-Ordovician age. Such index fossils must be distinctive, be globally distributed and have a short time range to be useful. However, misleading results are produced if the index fossils turn out to have longer fossil ranges than first thought.[50] Stratigraphy and biostratigraphy can in general provide only relative dating (A was before B), which is often sufficient for studying evolution. However, this is difficult for some time periods, because of the problems involved in matching up rocks of the same age across different continents.[51]
Family-tree relationships may also help to narrow down the date when lineages first appeared. For instance, if fossils of B or C date to X million years ago and the calculated "family tree" says A was an ancestor of B and C, then A must have evolved more than X million years ago.
It is also possible to estimate how long ago two living clades diverged – i.e. approximately how long ago their last common ancestor must have lived – by assuming that DNA mutations accumulate at a constant rate. These "molecular clocks", however, are fallible, and provide only a very approximate timing: for example, they are not sufficiently precise and reliable for estimating when the groups that feature in the Cambrian explosion first evolved,[52] and estimates produced by different techniques may vary by a factor of two.[15]
History of life

Earth formed about 4,570 million years ago and, after a collision that formed the Moon about 40 million years later, may have cooled quickly enough to have oceans and an atmosphere about 4,440 million years ago.[54] There is evidence on the Moon of a Late Heavy Bombardment by asteroids from 4,000 to 3,800 million years ago. If, as seems likely, such a bombardment struck Earth at the same time, the first atmosphere and oceans may have been stripped away.[55]
Paleontology traces the evolutionary history of life back to over 3,000 million years ago, possibly as far as 3,800 million years ago.[56] The oldest clear evidence of life on Earth dates to 3,000 million years ago, although there have been reports, often disputed, of fossil bacteria from 3,400 million years ago and of geochemical evidence for the presence of life 3,800 million years ago.[13][57] Some scientists have proposed that life on Earth was "seeded" from elsewhere,[58] but most research concentrates on various explanations of how life could have arisen independently on Earth.[59]
For about 2,000 million years microbial mats, multi-layered colonies of different bacteria, were the dominant life on Earth.[60] The evolution of oxygenic photosynthesis enabled them to play the major role in the oxygenation of the atmosphere[61] from about 2,400 million years ago. This change in the atmosphere increased their effectiveness as nurseries of evolution.[62] While eukaryotes, cells with complex internal structures, may have been present earlier, their evolution speeded up when they acquired the ability to transform oxygen from a poison to a powerful source of metabolic energy. This innovation may have come from primitive eukaryotes capturing oxygen-powered bacteria as endosymbionts and transforming them into organelles called mitochondria.[56][63] The earliest evidence of complex eukaryotes with organelles (such as mitochondria) dates from 1,850 million years ago.[27]

Multicellular life is composed only of eukaryotic cells, and the earliest evidence for it is the Francevillian Group Fossils from 2,100 million years ago,[64] although specialisation of cells for different functions first appears between 1,430 million years ago (a possible fungus) and 1,200 million years ago (a probable red alga). Sexual reproduction may be a prerequisite for specialisation of cells, as an asexual multicellular organism might be at risk of being taken over by rogue cells that retain the ability to reproduce.[65][66]
The earliest known animals are cnidarians from about 580 million years ago, but these are so modern-looking that must be descendants of earlier animals.[67] Early fossils of animals are rare because they had not developed mineralised, easily fossilized hard parts until about 548 million years ago.[68] The earliest modern-looking bilaterian animals appear in the Early Cambrian, along with several "weird wonders" that bear little obvious resemblance to any modern animals. There is a long-running debate about whether this Cambrian explosion was truly a very rapid period of evolutionary experimentation; alternative views are that modern-looking animals began evolving earlier but fossils of their precursors have not yet been found, or that the "weird wonders" are evolutionary "aunts" and "cousins" of modern groups.[69] Vertebrates remained a minor group until the first jawed fish appeared in the Late Ordovician.[70][71]

The spread of animals and plants from water to land required organisms to solve several problems, including protection against drying out and supporting themselves against gravity.[73][74][75][76] The earliest evidence of land plants and land invertebrates date back to about 476 million years ago and 490 million years ago respectively.[75][77] Those invertebrates, as indicated by their trace and body fossils, were shown to be arthropods known as euthycarcinoids.[78] The lineage that produced land vertebrates evolved later but very rapidly between 370 million years ago and 360 million years ago;[79] recent discoveries have overturned earlier ideas about the history and driving forces behind their evolution.[80] Land plants were so successful that their detritus caused an ecological crisis in the Late Devonian, until the evolution of fungi that could digest dead wood.[29]
During the Permian period, synapsids, including the ancestors of mammals, may have dominated land environments,[82] but this ended with the Permian–Triassic extinction event 251 million years ago, which came very close to wiping out all complex life.[83] The extinctions were apparently fairly sudden, at least among vertebrates.[84] During the slow recovery from this catastrophe a previously obscure group, archosaurs, became the most abundant and diverse terrestrial vertebrates. One archosaur group, the dinosaurs, were the dominant land vertebrates for the rest of the Mesozoic,[85] and birds evolved from one group of dinosaurs.[81] During this time mammals' ancestors survived only as small, mainly nocturnal insectivores, which may have accelerated the development of mammalian traits such as endothermy and hair.[86] After the Cretaceous–Paleogene extinction event 66 million years ago[87] killed off all the dinosaurs except the birds, mammals increased rapidly in size and diversity, and some took to the air and the sea.[88][89][90]
Fossil evidence indicates that flowering plants appeared and rapidly diversified in the Early Cretaceous between 130 million years ago and 90 million years ago.[91] Their rapid rise to dominance of terrestrial ecosystems is thought to have been propelled by coevolution with pollinating insects.[92] Social insects appeared around the same time and, although they account for only small parts of the insect "family tree", now form over 50% of the total mass of all insects.[93]
Humans evolved from a lineage of upright-walking apes whose earliest fossils date from over 6 million years ago.[94] Although early members of this lineage had chimp-sized brains, about 25% as big as modern humans', there are signs of a steady increase in brain size after about 3 million years ago.[95] There is a long-running debate about whether modern humans are descendants of a single small population in Africa, which then migrated all over the world less than 200,000 years ago and replaced previous hominine species, or arose worldwide at the same time as a result of interbreeding.[96]
Mass extinctions
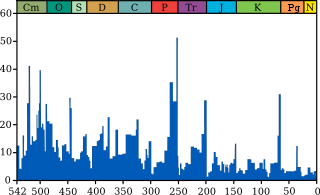
Life on earth has suffered occasional mass extinctions at least since 542 million years ago. Despite their disastrous effects, mass extinctions have sometimes accelerated the evolution of life on earth. When dominance of an ecological niche passes from one group of organisms to another, this is rarely because the new dominant group outcompetes the old, but usually because an extinction event allows new group to outlive the old and move into its niche.[97][98]
The fossil record appears to show that the rate of extinction is slowing down, with both the gaps between mass extinctions becoming longer and the average and background rates of extinction decreasing. However, it is not certain whether the actual rate of extinction has altered, since both of these observations could be explained in several ways:[99]
- The oceans may have become more hospitable to life over the last 500 million years and less vulnerable to mass extinctions: dissolved oxygen became more widespread and penetrated to greater depths; the development of life on land reduced the run-off of nutrients and hence the risk of eutrophication and anoxic events; marine ecosystems became more diversified so that food chains were less likely to be disrupted.[100][101]
- Reasonably complete fossils are very rare: most extinct organisms are represented only by partial fossils, and complete fossils are rarest in the oldest rocks. So paleontologists have mistakenly assigned parts of the same organism to different genera, which were often defined solely to accommodate these finds – the story of Anomalocaris is an example of this.[102] The risk of this mistake is higher for older fossils because these are often unlike parts of any living organism. Many "superfluous" genera are represented by fragments that are not found again, and these "superfluous" genera are interpreted as becoming extinct very quickly.[99]
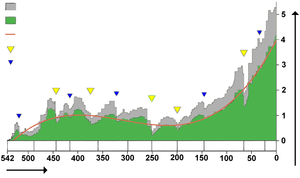
Biodiversity in the fossil record, which is
- "the number of distinct genera alive at any given time; that is, those whose first occurrence predates and whose last occurrence postdates that time"[103]
shows a different trend: a fairly swift rise from 542 to 400 million years ago, a slight decline from 400 to 200 million years ago, in which the devastating Permian–Triassic extinction event is an important factor, and a swift rise from 200 million years ago to the present.[103]
History
Although paleontology became established around 1800, earlier thinkers had noticed aspects of the fossil record. The ancient Greek philosopher Xenophanes (570–480 BC) concluded from fossil sea shells that some areas of land were once under water.[104] During the Middle Ages the Persian naturalist Ibn Sina, known as Avicenna in Europe, discussed fossils and proposed a theory of petrifying fluids on which Albert of Saxony elaborated in the 14th century.[105] The Chinese naturalist Shen Kuo (1031–1095) proposed a theory of climate change based on the presence of petrified bamboo in regions that in his time were too dry for bamboo.[106]
In early modern Europe, the systematic study of fossils emerged as an integral part of the changes in natural philosophy that occurred during the Age of Reason. In the Italian Renaissance, Leonardo Da Vinci made various significant contributions to the field as well depicted numerous fossils. Leonardo's contributions are central to the history of paleontology because he established a line of continuity between the two main branches of paleontology—ichnology and body fossil paleontology.[107][108][109] He identified the following:[107]
- The biogenic nature of ichnofossils, i.e. ichnofossils were structures left by living organisms;
- The utility of ichnofossils as paleoenvironmental tools—certain ichnofossils show the marine origin of rock strata;
- The importance of the neoichnological approach—recent traces are a key to understanding ichnofossils;
- The independence and complementary evidence of ichnofossils and body fossils—ichnofossils are distinct from body fossils, but can be integrated with body fossils to provide paleontological information
At the end of the 18th century Georges Cuvier's work established comparative anatomy as a scientific discipline and, by proving that some fossil animals resembled no living ones, demonstrated that animals could become extinct, leading to the emergence of paleontology.[110] The expanding knowledge of the fossil record also played an increasing role in the development of geology, particularly stratigraphy.[111]
The first half of the 19th century saw geological and paleontological activity become increasingly well organised with the growth of geologic societies and museums[112][113] and an increasing number of professional geologists and fossil specialists. Interest increased for reasons that were not purely scientific, as geology and paleontology helped industrialists to find and exploit natural resources such as coal.[114]
This contributed to a rapid increase in knowledge about the history of life on Earth and to progress in the definition of the geologic time scale, largely based on fossil evidence. In 1822 Henri Marie Ducrotay de Blanville, editor of Journal de Physique, coined the word "palaeontology" to refer to the study of ancient living organisms through fossils.[115] As knowledge of life's history continued to improve, it became increasingly obvious that there had been some kind of successive order to the development of life. This encouraged early evolutionary theories on the transmutation of species.[116] After Charles Darwin published Origin of Species in 1859, much of the focus of paleontology shifted to understanding evolutionary paths, including human evolution, and evolutionary theory.[116]

The last half of the 19th century saw a tremendous expansion in paleontological activity, especially in North America.[118] The trend continued in the 20th century with additional regions of the Earth being opened to systematic fossil collection. Fossils found in China near the end of the 20th century have been particularly important as they have provided new information about the earliest evolution of animals, early fish, dinosaurs and the evolution of birds.[119] The last few decades of the 20th century saw a renewed interest in mass extinctions and their role in the evolution of life on Earth.[120] There was also a renewed interest in the Cambrian explosion that apparently saw the development of the body plans of most animal phyla. The discovery of fossils of the Ediacaran biota and developments in paleobiology extended knowledge about the history of life back far before the Cambrian.[69]
Increasing awareness of Gregor Mendel's pioneering work in genetics led first to the development of population genetics and then in the mid-20th century to the modern evolutionary synthesis, which explains evolution as the outcome of events such as mutations and horizontal gene transfer, which provide genetic variation, with genetic drift and natural selection driving changes in this variation over time.[121] Within the next few years the role and operation of DNA in genetic inheritance were discovered, leading to what is now known as the "Central Dogma" of molecular biology.[122] In the 1960s molecular phylogenetics, the investigation of evolutionary "family trees" by techniques derived from biochemistry, began to make an impact, particularly when it was proposed that the human lineage had diverged from apes much more recently than was generally thought at the time.[123] Although this early study compared proteins from apes and humans, most molecular phylogenetics research is now based on comparisons of RNA and DNA.[124]
See also
- Biostratigraphy
- European land mammal age
- Fossil collecting – collecting fossils to study, collect or sell
- List of fossil sites – A table of worldwide localities notable for the presence of fossils (with link directory)
- List of notable fossils
- List of paleontologists – Wikimedia list article
- List of transitional fossils – Wikipedia list article
- Paleoanthropology – Study of ancient humans
- Paleobotany
- Paleogenetics
- Paleontographer
- Paleophycology – The study and identification of fossil algae
- Radiometric dating – Technique used to date materials such as rocks or carbon
- Taxonomy of commonly fossilised invertebrates
- Treatise on Invertebrate Paleontology – 1953 geology book
References
- "paleontology". Online Etymology Dictionary. Archived from the original on 2013-03-07.
- Cowen, R. (2000). History of Life (3rd ed.). Blackwell Science. p. xi. ISBN 0-632-04444-6.
- Laporte, L.F. (October 1988). "What, after All, Is Paleontology?". PALAIOS. 3 (5): 453. Bibcode:1988Palai...3..453L. doi:10.2307/3514718. JSTOR 3514718.
- Laudan, R. (1992). "What's so Special about the Past?". In Nitecki, M.H.; Nitecki, D.V. (eds.). History and Evolution. SUNY Press. p. 58. ISBN 0-7914-1211-3.
To structure my discussion of the historical sciences, I shall borrow a way of analyzing them from the great Victorian philosopher of science, William Whewell [...]. [...] while his analysis of the historical sciences (or as Whewell termed them, the palaetiological sciences) will doubtless need to be modified, it provides a good starting point. Among them he numbered geology, paleontology, cosmogony, philology, and what we would term archaeology and history.
- Cleland, C.E. (September 2002). "Methodological and Epistemic Differences between Historical Science and Experimental Science". Philosophy of Science. 69 (3): 474–496. doi:10.1086/342453. Archived from the original on October 3, 2008. Retrieved September 17, 2008.
- Laudan, R. (1992). "What's so Special about the Past?". In Nitecki, M.H.; Nitecki, D.V. (eds.). History and Evolution. SUNY Press. p. 58. ISBN 0-7914-1211-3.
[Whewell] distinguished three tasks for such a historical science (1837 [...]): ' the Description of the facts and phenomena; - the general Theory of the causes of change appropriate to the case; - and the Application of the theory to the facts.'
-
Perreault, Charles (2019). "The Search for Smoking Guns". The Quality of the Archaeological Record. Chicago: University of Chicago Press. p. 5. ISBN 9780226631011. Retrieved 9 January 2020.
Historical scientists successfully learn about the past by employing a 'smoking-gun' approach. They start by formulating multiple, mutually exclusive hypotheses and then search for a “smoking gun” that discriminates between these hypotheses [...].
-
"'Historical science' vs. 'experimental science'". National Center for Science Education. 25 October 2019. Retrieved 9 January 2020.
Philosophers of science draw a distinction between research directed towards identifying laws and research which seeks to determine how particular historical events occurred. They do not claim, however, that the line between these sorts of science can be drawn neatly, and certainly do not agree that historical claims are any less empirically verifiable than other sorts of claims. [...] 'we can separate their two enterprises by distinguishing means from ends. The astronomer's problem is a historical one because the goal is to infer the properties of a particular object; the astronomer uses laws only as a means. Particle physics, on the other hand, is a nomothetic discipline because the goal is to infer general laws; descriptions of particular objects are only relevant as a means.'
- "paleontology | science". Encyclopædia Britannica. Archived from the original on 2017-08-24. Retrieved 2017-08-24.
- McGraw-Hill Encyclopedia of Science & Technology. McGraw-Hill. 2002. p. 58. ISBN 0-07-913665-6.
- Laudan, R. (1992). "What's so Special about the Past?". In Nitecki, M.H.; Nitecki, D.V. (eds.). History and Evolution. SUNY Press. p. 57. ISBN 0-7914-1211-3.
- "How does paleontology differ from anthropology and archaeology?". University of California Museum of Paleontology. Archived from the original on September 16, 2008. Retrieved September 17, 2008.
- Brasier, M.; McLoughlin, N.; Green, O. & Wacey, D. (June 2006). "A fresh look at the fossil evidence for early Archaean cellular life" (PDF). Philosophical Transactions of the Royal Society B. 361 (1470): 887–902. doi:10.1098/rstb.2006.1835. PMC 1578727. PMID 16754605. Archived (PDF) from the original on September 11, 2008. Retrieved August 30, 2008.
- Twitchett RJ; Looy CV; Morante R; Visscher H; Wignall PB (2001). "Rapid and synchronous collapse of marine and terrestrial ecosystems during the end-Permian biotic crisis". Geology. 29 (4): 351–354. Bibcode:2001Geo....29..351T. doi:10.1130/0091-7613(2001)029<0351:RASCOM>2.0.CO;2.
- Peterson, Kevin J. & Butterfield, N.J. (2005). "Origin of the Eumetazoa: Testing ecological predictions of molecular clocks against the Proterozoic fossil record". Proceedings of the National Academy of Sciences. 102 (27): 9547–52. Bibcode:2005PNAS..102.9547P. doi:10.1073/pnas.0503660102. PMC 1172262. PMID 15983372.
- Hutchinson, J. R. & Garcia, M. (28 February 2002). "Tyrannosaurus was not a fast runner". Nature. 415 (6875): 1018–1021. Bibcode:2002Natur.415.1018H. doi:10.1038/4151018a. PMID 11875567. Summary in press release No Olympian: Analysis hints T. rex ran slowly, if at all Archived 2008-04-15 at the Wayback Machine
- Meers, M.B. (August 2003). "Maximum bite force and prey size of Tyrannosaurus rex and their relationships to the inference of feeding behavior". Historical Biology. 16 (1): 1–12. doi:10.1080/0891296021000050755.
- "The Four Winged Dinosaur: Wind Tunnel Test". NOVA. Retrieved June 5, 2010.
- Garwood, Russell J.; Rahman, Imran A.; Sutton, Mark D. A. (2010). "From clergymen to computers: the advent of virtual palaeontology". Geology Today. 26 (3): 96–100. doi:10.1111/j.1365-2451.2010.00753.x. Retrieved June 16, 2015.
- Mark Sutton; Imran Rahman; Russell Garwood (23 October 2013). Techniques for Virtual Palaeontology. Wiley. ISBN 978-1-118-59125-3.
- Bruner, Emiliano (November 2004). "Geometric morphometrics and palaeoneurology: brain shape evolution in the genus Homo". Journal of Human Evolution. 47 (5): 279–303. doi:10.1016/j.jhevol.2004.03.009. PMID 15530349.
- Cady, S.L. (April 1998). "Astrobiology: A New Frontier for 21st Century Paleontologists". PALAIOS. 13 (2): 95–97. Bibcode:1998Palai..13...95C. doi:10.2307/3515482. JSTOR 3515482. PMID 11542813.
- Plotnick, R.E. "A Somewhat Fuzzy Snapshot of Employment in Paleontology in the United States". Palaeontologia Electronica. Coquina Press. 11 (1). ISSN 1094-8074. Archived from the original on May 18, 2008. Retrieved September 17, 2008.
- "What is Paleontology?". University of California Museum of Paleontology. Archived from the original on August 3, 2008. Retrieved September 17, 2008.
- Kitchell, J.A. (1985). "Evolutionary Paleocology: Recent Contributions to Evolutionary Theory". Paleobiology. 11 (1): 91–104. doi:10.1017/S0094837300011428. Archived from the original on August 3, 2008. Retrieved September 17, 2008.
- Hoehler, T.M.; Bebout, B.M. & Des Marais, D.J. (19 July 2001). "The role of microbial mats in the production of reduced gases on the early Earth". Nature. 412 (6844): 324–327. Bibcode:2001Natur.412..324H. doi:10.1038/35085554. PMID 11460161.
- Hedges, S.B.; Blair, J.E; Venturi, M.L. & Shoe, J.L. (January 2004). "A molecular timescale of eukaryote evolution and the rise of complex multicellular life". BMC Evolutionary Biology. 4: 2. doi:10.1186/1471-2148-4-2. PMC 341452. PMID 15005799.
- "Paleoclimatology". Ohio State University. Archived from the original on November 9, 2007. Retrieved September 17, 2008.
- Algeo, T.J. & Scheckler, S.E. (1998). "Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events". Philosophical Transactions of the Royal Society B. 353 (1365): 113–130. doi:10.1098/rstb.1998.0195. PMC 1692181.
- "Biostratigraphy: William Smith". Archived from the original on July 24, 2008. Retrieved September 17, 2008.
- "Biogeography: Wallace and Wegener (1 of 2)". University of California Museum of Paleontology and University of California at Berkeley. Archived from the original on May 15, 2008. Retrieved September 17, 2008.
- "What is paleontology?". University of California Museum of Paleontology. Archived from the original on September 16, 2008. Retrieved September 17, 2008.
- Benton MJ; Wills MA; Hitchin R (2000). "Quality of the fossil record through time". Nature. 403 (6769): 534–7. Bibcode:2000Natur.403..534B. doi:10.1038/35000558. PMID 10676959.
- Non-technical summary Archived 2007-08-09 at the Wayback Machine
- Butterfield, N.J. (2003). "Exceptional Fossil Preservation and the Cambrian Explosion". Integrative and Comparative Biology. 43 (1): 166–177. doi:10.1093/icb/43.1.166. PMID 21680421.
- Cowen, R. (2000). History of Life (3rd ed.). Blackwell Science. p. 61. ISBN 0-632-04444-6.
- Butterfield, N.J. (2001). "Ecology and evolution of Cambrian plankton". The Ecology of the Cambrian Radiation. New York: Columbia University Press: 200–216. Retrieved September 27, 2007.
- Signor, P.W. (1982). "Sampling bias, gradual extinction patterns and catastrophes in the fossil record". Geological Implications of Impacts of Large Asteroids and Comets on the Earth. Geological Society of America Special Papers. Boulder, CO: Geological Society of America. 190: 291–296. doi:10.1130/SPE190-p291. ISBN 0-8137-2190-3. A 84–25651 10–42. Retrieved January 1, 2008.
- Fedonkin, M.A.; Gehling, J.G.; Grey, K.; Narbonne, G.M.; Vickers-Rich, P. (2007). The Rise of Animals: Evolution and Diversification of the Kingdom Animalia. JHU Press. pp. 213–216. ISBN 978-0-8018-8679-9.
- e.g. Seilacher, A. (1994). "How valid is Cruziana Stratigraphy?". International Journal of Earth Sciences. 83 (4): 752–758. Bibcode:1994GeoRu..83..752S. doi:10.1007/BF00251073.
- Brocks, J.J.; Logan, G.A.; Buick, R. & Summons, R.E. (1999). "Archaean molecular fossils and the rise of eukaryotes". Science. 285 (5430): 1033–1036. doi:10.1126/science.285.5430.1033. PMID 10446042.
- Brochu, C.A & Sumrall, C.D. (July 2001). "Phylogenetic Nomenclature and Paleontology". Journal of Paleontology. 75 (4): 754–757. doi:10.1666/0022-3360(2001)075<0754:PNAP>2.0.CO;2. ISSN 0022-3360. JSTOR 1306999.
- Ereshefsky, M. (2001). The Poverty of the Linnaean Hierarchy: A Philosophical Study of Biological Taxonomy. Cambridge University Press. p. 5. ISBN 0-521-78170-1.
- Cowen, R. (2000). History of Life (3rd ed.). Blackwell Science. pp. 47–50. ISBN 0-632-04444-6.
- Garwood, Russell J.; Sharma, Prashant P.; Dunlop, Jason A.; Giribet, Gonzalo (2014). "A Paleozoic Stem Group to Mite Harvestmen Revealed through Integration of Phylogenetics and Development". Current Biology. 24 (9): 1017–1023. doi:10.1016/j.cub.2014.03.039. PMID 24726154. Retrieved April 17, 2014.
- Cohen, B. L.; Holmer, L. E. & Luter, C. (2003). "The brachiopod fold: a neglected body plan hypothesis" (PDF). Palaeontology. 46 (1): 59–65. doi:10.1111/1475-4983.00287. Archived from the original (PDF) on October 3, 2008. Retrieved August 7, 2008.
- Martin, M.W.; Grazhdankin, D.V.; Bowring, S.A.; Evans, D.A.D.; Fedonkin, M.A.; Kirschvink, J.L. (May 5, 2000). "Age of Neoproterozoic Bilaterian Body and Trace Fossils, White Sea, Russia: Implications for Metazoan Evolution". Science (abstract). 288 (5467): 841–5. Bibcode:2000Sci...288..841M. doi:10.1126/science.288.5467.841. PMID 10797002.
- Pufahl, P.K.; Grimm, K.A.; Abed, A.M. & Sadaqah, R.M.Y. (October 2003). "Upper Cretaceous (Campanian) phosphorites in Jordan: implications for the formation of a south Tethyan phosphorite giant". Sedimentary Geology. 161 (3–4): 175–205. Bibcode:2003SedG..161..175P. doi:10.1016/S0037-0738(03)00070-8.
- "Geologic Time: Radiometric Time Scale". U.S. Geological Survey. Archived from the original on September 21, 2008. Retrieved September 20, 2008.
- Löfgren, A. (2004). "The conodont fauna in the Middle Ordovician Eoplacognathus pseudoplanus Zone of Baltoscandia". Geological Magazine. 141 (4): 505–524. Bibcode:2004GeoM..141..505L. doi:10.1017/S0016756804009227.
- Gehling, James; Jensen, Sören; Droser, Mary; Myrow, Paul; Narbonne, Guy (March 2001). "Burrowing below the basal Cambrian GSSP, Fortune Head, Newfoundland". Geological Magazine. 138 (2): 213–218. Bibcode:2001GeoM..138..213G. doi:10.1017/S001675680100509X.
- e.g. Gehling, James; Jensen, Sören; Droser, Mary; Myrow, Paul; Narbonne, Guy (March 2001). "Burrowing below the basal Cambrian GSSP, Fortune Head, Newfoundland". Geological Magazine. 138 (2): 213–218. Bibcode:2001GeoM..138..213G. doi:10.1017/S001675680100509X.
- Hug, L.A. & Roger, A.J. (2007). "The Impact of Fossils and Taxon Sampling on Ancient Molecular Dating Analyses". Molecular Biology and Evolution. 24 (8): 889–1897. doi:10.1093/molbev/msm115. PMID 17556757.
- Manten, A.A. (1966). "Some problematic shallow-marine structures". Marine Geol. 4 (3): 227–232. Bibcode:1966MGeol...4..227M. doi:10.1016/0025-3227(66)90023-5. hdl:1874/16526. Archived from the original on October 21, 2008. Retrieved June 18, 2007.
-
- "Early Earth Likely Had Continents And Was Habitable". 2005-11-17. Archived from the original on 2008-10-14.
* Cavosie, A. J.; J. W. Valley, S. A., Wilde & E.I.M.F. (July 15, 2005). "Magmatic δ18O in 4400–3900 Ma detrital zircons: A record of the alteration and recycling of crust in the Early Archean". Earth and Planetary Science Letters. 235 (3–4): 663–681. Bibcode:2005E&PSL.235..663C. doi:10.1016/j.epsl.2005.04.028.CS1 maint: multiple names: authors list (link)
- "Early Earth Likely Had Continents And Was Habitable". 2005-11-17. Archived from the original on 2008-10-14.
- Dauphas, N.; Robert, F. & Marty, B. (December 2000). "The Late Asteroidal and Cometary Bombardment of Earth as Recorded in Water Deuterium to Protium Ratio". Icarus. 148 (2): 508–512. Bibcode:2000Icar..148..508D. doi:10.1006/icar.2000.6489.
- Garwood, Russell J. (2012). "Patterns In Palaeontology: The first 3 billion years of evolution". Palaeontology Online. 2 (11): 1–14. Archived from the original on June 26, 2015. Retrieved June 25, 2015.
- Schopf, J. (2006). "Fossil evidence of Archaean life". Philos Trans R Soc Lond B Biol Sci. 361 (1470): 869–85. doi:10.1098/rstb.2006.1834. PMC 1578735. PMID 16754604.
-
- Arrhenius, S. (1903). "The Propagation of Life in Space". Die Umschau. 7: 32. Bibcode:1980qel..book...32A. Reprinted in Goldsmith, D. (ed.). The Quest for Extraterrestrial Life. University Science Books. ISBN 0-19-855704-3.
* Hoyle, F. & Wickramasinghe, C. (1979). "On the Nature of Interstellar Grains". Astrophysics and Space Science. 66 (1): 77–90. Bibcode:1979Ap&SS..66...77H. doi:10.1007/BF00648361.
* Crick, F. H.; Orgel, L. E. (1973). "Directed Panspermia". Icarus. 19 (3): 341–348. Bibcode:1973Icar...19..341C. doi:10.1016/0019-1035(73)90110-3.
- Arrhenius, S. (1903). "The Propagation of Life in Space". Die Umschau. 7: 32. Bibcode:1980qel..book...32A. Reprinted in Goldsmith, D. (ed.). The Quest for Extraterrestrial Life. University Science Books. ISBN 0-19-855704-3.
- Peretó, J. (2005). "Controversies on the origin of life" (PDF). Int. Microbiol. 8 (1): 23–31. PMID 15906258. Archived from the original (PDF) on August 24, 2015. Retrieved October 7, 2007.
- Krumbein, W.E.; Brehm, U.; Gerdes, G.; Gorbushina, A.A.; Levit, G. & Palinska, K.A. (2003). "Biofilm, Biodictyon, Biomat Microbialites, Oolites, Stromatolites, Geophysiology, Global Mechanism, Parahistology". In Krumbein, W.E.; Paterson, D.M. & Zavarzin, G.A. (eds.). Fossil and Recent Biofilms: A Natural History of Life on Earth (PDF). Kluwer Academic. pp. 1–28. ISBN 1-4020-1597-6. Archived from the original (PDF) on January 6, 2007. Retrieved July 9, 2008.
- Hoehler, T.M.; Bebout, B.M. & Des Marais, D.J. (July 19, 2001). "The role of microbial mats in the production of reduced gases on the early Earth". Nature. 412 (6844): 324–327. Bibcode:2001Natur.412..324H. doi:10.1038/35085554. PMID 11460161.
- Nisbet, E.G. & Fowler, C.M.R. (December 7, 1999). "Archaean metabolic evolution of microbial mats". Proceedings of the Royal Society B. 266 (1436): 2375. doi:10.1098/rspb.1999.0934. PMC 1690475.
- Gray MW; Burger G; Lang BF (March 1999). "Mitochondrial evolution". Science. 283 (5407): 1476–81. Bibcode:1999Sci...283.1476G. doi:10.1126/science.283.5407.1476. PMID 10066161.
- El Albani, Abderrazak; Bengtson, Stefan; Canfield, Donald E.; Bekker, Andrey; Macchiarelli, Reberto; Mazurier, Arnaud; Hammarlund, Emma U.; Boulvais, Philippe; et al. (July 2010). "Large colonial organisms with coordinated growth in oxygenated environments 2.1 Gyr ago". Nature. 466 (7302): 100–104. Bibcode:2010Natur.466..100A. doi:10.1038/nature09166. PMID 20596019.
- Butterfield, N.J. (September 2000). "Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology. 26 (3): 386–404. doi:10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. ISSN 0094-8373. Archived from the original on 2007-03-07. Retrieved 2008-09-02.
- Butterfield, N.J. (2005). "Probable Proterozoic fungi". Paleobiology. 31 (1): 165–182. doi:10.1666/0094-8373(2005)031<0165:PPF>2.0.CO;2. ISSN 0094-8373. Archived from the original on 2009-01-29. Retrieved 2008-09-02.
- Chen, J.-Y.; Oliveri, P.; Gao, F.; Dornbos, S.Q.; Li, C-W.; Bottjer, D.J. & Davidson, E.H. (August 2002). "Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China" (PDF). Developmental Biology. 248 (1): 182–196. doi:10.1006/dbio.2002.0714. PMID 12142030. Archived from the original (PDF) on September 11, 2008. Retrieved September 3, 2008.
- Bengtson, S. (2004). Lipps, J.H.; Waggoner, B.M. (eds.). "Early Skeletal Fossils" (PDF). The Paleontological Society Papers. 10 Neoproterozoic—Cambrian Biological Revolutions: 67–78. doi:10.1017/S1089332600002345. Archived from the original (PDF) on 2017-02-11. Retrieved July 18, 2008.
- Marshall, C.R. (2006). "Explaining the Cambrian "Explosion" of Animals". Annu. Rev. Earth Planet. Sci. 34: 355–384. Bibcode:2006AREPS..34..355M. doi:10.1146/annurev.earth.33.031504.103001.
- Conway Morris, S. (August 2, 2003). "Once we were worms". New Scientist. 179 (2406): 34. Archived from the original on July 25, 2008. Retrieved September 5, 2008.
- Sansom I.J., Smith, M.M. & Smith, M.P. (2001). "The Ordovician radiation of vertebrates". In Ahlberg, P.E. (ed.). Major Events in Early Vertebrate Evolution. Taylor and Francis. pp. 156–171. ISBN 0-415-23370-4.CS1 maint: multiple names: authors list (link)
- Luo, Z.; Chen, P.; Li, G. & Chen, M. (March 2007). "A new eutriconodont mammal and evolutionary development in early mammals". Nature. 446 (7133): 288–293. Bibcode:2007Natur.446..288L. doi:10.1038/nature05627. PMID 17361176.
- Russell Garwood & Gregory Edgecombe (2011). "Early terrestrial animals, evolution and uncertainty". Evolution: Education and Outreach. 4 (3): 489–501. doi:10.1007/s12052-011-0357-y.
- Selden, P.A. (2001). "Terrestrialization of Animals". In Briggs, D.E.G.; Crowther, P.R. (eds.). Palaeobiology II: A Synthesis. Blackwell. pp. 71–74. ISBN 0-632-05149-3.
- Kenrick, P. & Crane, P.R. (September 1997). "The origin and early evolution of plants on land" (PDF). Nature. 389 (6646): 33. Bibcode:1997Natur.389...33K. doi:10.1038/37918. Archived (PDF) from the original on 2010-12-17.
- Laurin, M. (2010). How Vertebrates Left the Water. Berkeley, California, USA.: University of California Press. ISBN 978-0-520-26647-6.
- MacNaughton, R.B.; Cole, J.M.; Dalrymple, R.W.; Braddy, S.J.; Briggs, D.E.G. & Lukie, T.D. (May 2002). "First steps on land: Arthropod trackways in Cambrian-Ordovician eolian sandstone, southeastern Ontario, Canada". Geology. 30 (5): 391–394. Bibcode:2002Geo....30..391M. doi:10.1130/0091-7613(2002)030<0391:FSOLAT>2.0.CO;2. ISSN 0091-7613.
- Collette, J.H.; Gass, K.C. & Hagadorn, J.W.. (May 2012). "Protichnites eremita unshelled? Experimental model-based neoichnology and new evidence for a euthycarcinoid affinity for this ichnospecies". Journal of Paleontology. 86 (3): 442–454. doi:10.1666/11-056.1.
- Gordon, M.S; Graham, J.B. & Wang, T. (September–October 2004). "Revisiting the Vertebrate Invasion of the Land". Physiological and Biochemical Zoology. 77 (5): 697–699. doi:10.1086/425182.
- Clack, J.A. (November 2005). "Getting a Leg Up on Land". Scientific American. Retrieved September 6, 2008.
- Padian, Kevin. (2004). "Basal Avialae". In Weishampel, David B.; Dodson, Peter; Osmólska, Halszka (eds.). The Dinosauria (Second ed.). Berkeley: University of California Press. pp. 210–231. ISBN 0-520-24209-2.
- Sidor, C.A.; O'Keefe, F.R.; Damiani, R.; Steyer, J.S.; Smith, R.M.H.; Larsson, H.C.E.; Sereno, P.C.; Ide, O & Maga, A. (April 2005). "Permian tetrapods from the Sahara show climate-controlled endemism in Pangaea". Nature. 434 (7035): 886–889. Bibcode:2005Natur.434..886S. doi:10.1038/nature03393. PMID 15829962.
- Benton M.J. (2005). When Life Nearly Died: The Greatest Mass Extinction of All Time. Thames & Hudson. ISBN 978-0-500-28573-2.
- Ward, P.D.; Botha, J.; Buick, R.; Kock, M.O.; et al. (2005). "Abrupt and gradual extinction among late Permian land vertebrates in the Karoo Basin, South Africa" (PDF). Science. 307 (5710): 709–714. Bibcode:2005Sci...307..709W. doi:10.1126/science.1107068. PMID 15661973. Archived (PDF) from the original on 2012-08-13.
- Benton, M.J. (March 1983). "Dinosaur Success in the Triassic: a Noncompetitive Ecological Model" (PDF). Quarterly Review of Biology. 58 (1): 29–55. doi:10.1086/413056. Archived from the original (PDF) on September 11, 2008. Retrieved September 8, 2008.
- Ruben, J.A. & Jones, T.D. (2000). "Selective Factors Associated with the Origin of Fur and Feathers". American Zoologist. 40 (4): 585–596. doi:10.1093/icb/40.4.585.
- Renne, Paul R.; Deino, Alan L.; Hilgen, Frederik J.; Kuiper, Klaudia F.; Mark, Darren F.; Mitchell, William S.; Morgan, Leah E.; Mundil, Roland; Smit, Jan (7 February 2013). "Time Scales of Critical Events Around the Cretaceous-Paleogene Boundary". Science. 339 (6120): 684–687. Bibcode:2013Sci...339..684R. doi:10.1126/science.1230492. PMID 23393261.
- Alroy J. (March 1999). "The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation". Systematic Biology. 48 (1): 107–18. doi:10.1080/106351599260472. PMID 12078635.
- Simmons, N.B.; Seymour, K.L.; Habersetzer, J. & Gunnell, G.F. (February 2008). "Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation" (PDF). Nature. 451 (7180): 818–821. Bibcode:2008Natur.451..818S. doi:10.1038/nature06549. hdl:2027.42/62816. PMID 18270539.
- J. G. M. Thewissen; S. I. Madar & S. T. Hussain (1996). "Ambulocetus natans, an Eocene cetacean (Mammalia) from Pakistan". Courier Forschungsinstitut Senckenberg. 191: 1–86.
- Crane, P.R.; Friis, E.M. & Pedersen, K.R. (2000). "The Origin and Early Diversification of Angiosperms". In Gee, H. (ed.). Shaking the Tree: Readings from Nature in the History of Life. University of Chicago Press. pp. 233–250. ISBN 0-226-28496-4.
- Crepet, W.L. (November 2000). "Progress in understanding angiosperm history, success, and relationships: Darwin's abominably "perplexing phenomenon"". Proceedings of the National Academy of Sciences. 97 (24): 12939–12941. Bibcode:2000PNAS...9712939C. doi:10.1073/pnas.97.24.12939. PMC 34068. PMID 11087846.
- Brunet M., Guy; F., Pilbeam; D., Mackaye, H.T.; et al. (July 2002). "A new hominid from the Upper Miocene of Chad, Central Africa". Nature. 418 (6894): 145–151. Bibcode:2002Natur.418..145B. doi:10.1038/nature00879. PMID 12110880.CS1 maint: multiple names: authors list (link)
- De Miguel, C. & M. Henneberg, M. (2001). "Variation in hominid brain size: How much is due to method?". HOMO: Journal of Comparative Human Biology. 52 (1): 3–58. doi:10.1078/0018-442X-00019. PMID 11515396.
- Leakey, Richard (1994). The Origin of Humankind. Science Masters Series. New York, NY: Basic Books. pp. 87–89. ISBN 0-465-05313-0.
- Benton, M.J. (2004). "6. Reptiles Of The Triassic". Vertebrate Palaeontology. Blackwell. ISBN 0-04-566002-6. Retrieved November 17, 2008.
- Van Valkenburgh, B. (1999). "Major patterns in the history of xarnivorous mammals". Annual Review of Earth and Planetary Sciences. 27: 463–493. Bibcode:1999AREPS..27..463V. doi:10.1146/annurev.earth.27.1.463.
- MacLeod, Norman (2001-01-06). "Extinction!". Archived from the original on April 4, 2008. Retrieved September 11, 2008.
- Martin, R.E. (1995). "Cyclic and secular variation in microfossil biomineralization: clues to the biogeochemical evolution of Phanerozoic oceans". Global and Planetary Change. 11 (1): 1. Bibcode:1995GPC....11....1M. doi:10.1016/0921-8181(94)00011-2.
- Martin, R.E. (1996). "Secular increase in nutrient levels through the Phanerozoic: Implications for productivity, biomass, and diversity of the marine biosphere". PALAIOS. 11 (3): 209–219. Bibcode:1996Palai..11..209M. doi:10.2307/3515230. JSTOR 3515230.
- Gould, S.J. (1990). Wonderful Life: The Burgess Shale and the Nature of History. Hutchinson Radius. pp. 194–206. ISBN 0-09-174271-4.
- Rohde, R.A. & Muller, R.A. (March 2005). "Cycles in fossil diversity" (PDF). Nature. 434 (7030): 208–210. Bibcode:2005Natur.434..208R. doi:10.1038/nature03339. PMID 15758998. Archived (PDF) from the original on October 3, 2008. Retrieved September 22, 2008.
- Rudwick, Martin J.S. (1985). The Meaning of Fossils (2nd ed.). The University of Chicago Press. p. 39. ISBN 0-226-73103-0.
- Rudwick, Martin J.S. (1985). The Meaning of Fossils (2nd ed.). The University of Chicago Press. p. 24. ISBN 0-226-73103-0.
- Needham, Joseph (1986). Science and Civilization in China: Volume 3, Mathematics and the Sciences of the Heavens and the Earth. Caves Books Ltd. p. 614. ISBN 0-253-34547-2.
- Baucon, A. 2010. Leonardo da Vinci, the founding father of ichnology. Palaios 25. Abstract available from the author's webpage
- Baucon A., Bordy E., Brustur T., Buatois L., Cunningham T., De C., Duffin C., Felletti F., Gaillard C., Hu B., Hu L., Jensen S., Knaust D., Lockley M., Lowe P., Mayor A., Mayoral E., Mikulas R., Muttoni G., Neto de Carvalho C., Pemberton S., Pollard J., Rindsberg A., Santos A., Seike K., Song H., Turner S., Uchman A., Wang Y., Yi-ming G., Zhang L., Zhang W. 2012. A history of ideas in ichnology. In: Bromley R.G., Knaust D. Trace Fossils as Indicators of Sedimentary Environments. Developments in Sedimentology, vol. 64. Tracemaker.com
- Baucon, A. 2010. Da Vinci’s Paleodictyon: the fractal beauty of traces. Acta Geologica Polonica, 60(1). Accessible from the author's homepage
- McGowan, Christopher (2001). The Dragon Seekers. Persus Publishing. pp. 3–4. ISBN 0-7382-0282-7.
- Palmer, D. (2005). Earth Time: Exploring the Deep Past from Victorian England to the Grand Canyon. Wiley. ISBN 9780470022214.
- Greene, Marjorie; David Depew (2004). The Philosophy of Biology: An Episodic History. Cambridge University Press. pp. 128–130. ISBN 0-521-64371-6.
- Bowler, Peter J.; Iwan Rhys Morus (2005). Making Modern Science. The University of Chicago Press. pp. 168–169. ISBN 0-226-06861-7.
- Rudwick, Martin J. S. (1985). The Meaning of Fossils (2nd ed.). The University of Chicago Press. pp. 200–201. ISBN 0-226-73103-0.
- Rudwick, Martin J.S. (2008). Worlds Before Adam: The Reconstruction of Geohistory in the Age of Reform. The University of Chicago Press. p. 48. ISBN 978-0-226-73128-5.
- Buckland W & Gould SJ (1980). Geology and Mineralogy Considered With Reference to Natural Theology (History of Paleontology). Ayer Company Publishing. ISBN 978-0-405-12706-9.
- Shu, D. G.; Morris, S. C.; Han, J.; Zhang, Z. F.; Yasui, K.; Janvier, P.; Chen, L.; Zhang, X. L.; Liu, J. N.; Li, Y.; Liu, H. -Q. (2003), "Head and backbone of the Early Cambrian vertebrate Haikouichthys", Nature, 421 (6922): 526–529, Bibcode:2003Natur.421..526S, doi:10.1038/nature01264, PMID 12556891, archived from the original on 2015-11-24
- Everhart, Michael J. (2005). Oceans of Kansas: A Natural History of the Western Interior Sea. Indiana University Press. p. 17. ISBN 0-253-34547-2.
- Gee, H., ed. (2001). Rise of the Dragon: Readings from Nature on the Chinese Fossil Record. Chicago, Ill. ;London: University of Chicago Press. p. 276. ISBN 0-226-28491-3.
- Bowler, Peter J. (2003). Evolution:The History of an Idea. University of California Press. pp. 351–352. ISBN 0-520-23693-9.
- Bowler, Peter J. (2003). Evolution: The History of an Idea. University of California Press. pp. 325–339. ISBN 0-520-23693-9.
- Crick, F.H.C. (1955). "On degenerate templates and the adaptor hypothesis" (PDF). Archived from the original (PDF) on 2008-10-01. Retrieved 2008-10-04.
- Sarich, V.M. & Wilson, A.C. (December 1967). "Immunological time scale for hominid evolution". Science. 158 (3805): 1200–1203. Bibcode:1967Sci...158.1200S. doi:10.1126/science.158.3805.1200. PMID 4964406.
- Page, R.D.M. & Holmes, E.C. (1998). Molecular Evolution: A Phylogenetic Approach. Oxford: Blackwell Science. p. 2. ISBN 0-86542-889-1.
External links
- Smithsonian's Paleobiology website
- University of California Museum of Paleontology
- The Paleontological Society
- The Palaeontological Association
- The Society of Vertebrate Paleontology
- The Paleontology Portal
- "Geology, Paleontology & Theories of the Earth", a collection of more than 100 digitised landmark and early books on Earth sciences at the Linda Hall Library