Sexual reproduction
Sexual reproduction is a type of reproduction that involves a complex life cycle in which a gamete (such as a sperm or egg cell) with a single set of chromosomes (haploid) combines with another to produce an organism composed of cells with two sets of chromosomes (diploid).[1] Sexual reproduction is the most common life cycle in multicellular eukaryotes, such as animals, fungi and plants. Sexual reproduction does not occur in prokaryotes (organisms without cell nuclei), but they have processes with similar effects such as bacterial conjugation, transformation and transduction, which may have been precursors to sexual reproduction in early eukaryotes.
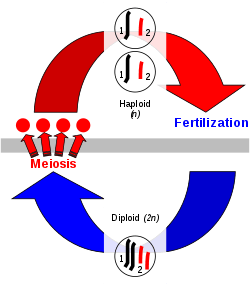
In the production of sex cells in eukaryotes, diploid mother cells divide to produce haploid cells known as gametes in a process called meiosis that involves genetic recombination. The homologous chromosomes pair up so that their DNA sequences are aligned with each other, and this is followed by exchange of genetic information between them. Two rounds of cell division then produce four haploid gametes, each with half the number of chromosomes from each parent cell, but with the genetic information in the parental chromosomes recombined. Two haploid gametes combine into one diploid cell known as a zygote in a process called fertilisation. The zygote incorporates genetic material from both gametes. Multiple cell divisions, without change of the number of chromosomes, then form a multicellular diploid phase or generation.
In human reproduction, each cell contains 46 chromosomes in 23 pairs. Meiosis in the parents' gonads produces gametes that each contain only 23 chromosomes that are genetic recombinants of the DNA sequences contained in the parental chromosomes. When the nuclei of the gametes come together to form a fertilized egg or zygote, each cell of the resulting child will have 23 chromosomes from each parent, or 46 in total.[2][3]
In plants only, the diploid phase, known as the sporophyte, produces spores by meiosis that germinate and then divide by mitosis to form a haploid multicellular phase, the gametophyte, that produces gametes directly by mitosis. This type of life cycle, involving alternation between two multicellular phases, the sexual haploid gametophyte and asexual diploid sporophyte, is known as alternation of generations.
The evolution of sexual reproduction is considered paradoxical,[3] because asexual reproduction should be able to outperform it as every young organism created can bear its own young. This implies that an asexual population has an intrinsic capacity to grow more rapidly with each generation.[4] This 50% cost is a fitness disadvantage of sexual reproduction.[5] The two-fold cost of sex includes this cost and the fact that any organism can only pass on 50% of its own genes to its offspring. One definite advantage of sexual reproduction is that it impedes the accumulation of genetic mutations.[6]
Sexual selection is a mode of natural selection in which some individuals out-reproduce others of a population because they are better at securing mates for sexual reproduction.[7][8] It has been described as "a powerful evolutionary force that does not exist in asexual populations."[9]
Evolution
The first fossilized evidence of sexual reproduction in eukaryotes is from the Stenian period, about 1 to 1.2 billion years ago.[10]
Biologists studying evolution propose several explanations for the development of sexual reproduction and its maintenance. These reasons include reducing the likelihood of the accumulation of deleterious mutations, increasing rate of adaptation to changing environments,[11] dealing with competition, DNA repair and masking deleterious mutations.[12][13][14] All of these ideas about why sexual reproduction has been maintained are generally supported, but ultimately the size of the population determines if sexual reproduction is entirely beneficial. Larger populations appear to respond more quickly to some of the benefits obtained through sexual reproduction than do smaller population sizes.[15]
Maintenance of sexual reproduction has been explained by theories that work at several levels of selection, though some of these models remain controversial. However, newer models presented in recent years suggest a basic advantage for sexual reproduction in slowly reproducing complex organisms.
Sexual reproduction allows these species to exhibit characteristics that depend on the specific environment that they inhabit, and the particular survival strategies that they employ.[16]
Sexual selection
In order to sexually reproduce, both males and females need to find a mate. Generally in animals mate choice is made by females while males compete to be chosen. This can lead organisms to extreme efforts in order to reproduce, such as combat and display, or produce extreme features caused by a positive feedback known as a Fisherian runaway. Thus sexual reproduction, as a form of natural selection, has an effect on evolution. Sexual dimorphism is where the basic phenotypic traits vary between males and females of the same species. Dimorphism is found in both sex organs and in secondary sex characteristics, body size, physical strength and morphology, biological ornamentation, behavior and other bodily traits. However, sexual selection is only implied over an extended period of time leading to sexual dimorphism.[17]
Sex ratio
Apart from some eusocial wasps, organisms which reproduce sexually have a 1:1 sex ratio of male and female births. The English statistician and biologist Ronald Fisher outlined why this is so in what has come to be known as Fisher's principle.[18] This essentially says the following:
- Suppose male births are less common than female.
- A newborn male then has better mating prospects than a newborn female, and therefore can expect to have more offspring.
- Therefore parents genetically disposed to produce males tend to have more than average numbers of grandchildren born to them.
- Therefore the genes for male-producing tendencies spread, and male births become more common.
- As the 1:1 sex ratio is approached, the advantage associated with producing males dies away.
- The same reasoning holds if females are substituted for males throughout. Therefore 1:1 is the equilibrium ratio.
Animals
Insects

Insect species make up more than two-thirds of all extant animal species. Most insect species reproduce sexually, though some species are facultatively parthenogenetic. Many insects species have sexual dimorphism, while in others the sexes look nearly identical. Typically they have two sexes with males producing spermatozoa and females ova. The ova develop into eggs that have a covering called the chorion, which forms before internal fertilization. Insects have very diverse mating and reproductive strategies most often resulting in the male depositing spermatophore within the female, which she stores until she is ready for egg fertilization. After fertilization, and the formation of a zygote, and varying degrees of development, in many species the eggs are deposited outside the female; while in others, they develop further within the female and are born live.
Birds
Mammals
There are three extant kinds of mammals: monotremes, placentals and marsupials, all with internal fertilization. In placental mammals, offspring are born as juveniles: complete animals with the sex organs present although not reproductively functional. After several months or years, depending on the species, the sex organs develop further to maturity and the animal becomes sexually mature. Most female mammals are only fertile during certain periods during their estrous cycle, at which point they are ready to mate. Individual male and female mammals meet and carry out copulation. For most mammals, males and females exchange sexual partners throughout their adult lives.[19][20][21]
Fish
The vast majority of fish species lay eggs that are then fertilized by the male.[22] Some species lay their eggs on a substrate like a rock or on plants, while others scatter their eggs and the eggs are fertilized as they drift or sink in the water column.
Some fish species use internal fertilization and then disperse the developing eggs or give birth to live offspring. Fish that have live-bearing offspring include the guppy and mollies or Poecilia. Fishes that give birth to live young can be ovoviviparous, where the eggs are fertilized within the female and the eggs simply hatch within the female body, or in seahorses, the male carries the developing young within a pouch, and gives birth to live young.[23] Fishes can also be viviparous, where the female supplies nourishment to the internally growing offspring. Some fish are hermaphrodites, where a single fish is both male and female and can produce eggs and sperm. In hermaphroditic fish, some are male and female at the same time while in other fish they are serially hermaphroditic; starting as one sex and changing to the other. In at least one hermaphroditic species, self-fertilization occurs when the eggs and sperm are released together. Internal self-fertilization may occur in some other species.[24] One fish species does not reproduce by sexual reproduction but uses sex to produce offspring; Poecilia formosa is a unisex species that uses a form of parthenogenesis called gynogenesis, where unfertilized eggs develop into embryos that produce female offspring. Poecilia formosa mate with males of other fish species that use internal fertilization, the sperm does not fertilize the eggs but stimulates the growth of the eggs which develops into embryos.[25]
Amphibians
Mollusks
Plants
Animals have life cycles with a single diploid multicellular phase that produces haploid gametes directly by meiosis. Male gametes are called sperm, and female gametes are called eggs or ova. In animals, fertilization of the ovum by a sperm results in the formation of a diploid zygote that develops by repeated mitotic divisions into a diploid adult. Plants have two multicellular life-cycle phases, resulting in an alternation of generations. Plant zygotes germinate and divide repeatedly by mitosis to produce a diploid multicellular organism known as the sporophyte. The mature sporophyte produces haploid spores by meiosis that germinate and divide by mitosis to form a multicellular gametophyte phase that produces gametes at maturity. The gametophytes of different groups of plants vary in size. Mosses and other pteridophytic plants may have gametophytes consisting of several million cells, while angiosperms have as few as three cells in each pollen grain.
Flowering plants

Flowering plants are the dominant plant form on land and they reproduce either sexually or asexually. Often their most distinguishing feature is their reproductive organs, commonly called flowers. The anther produces pollen grains which contain the male gametophytes that produce sperm nuclei. For pollination to occur, pollen grains must attach to the stigma of the female reproductive structure (carpel), where the female gametophytes (ovules) are located inside the ovary. After the pollen tube grows through the carpel's style, the sex cell nuclei from the pollen grain migrate into the ovule to fertilize the egg cell and endosperm nuclei within the female gametophyte in a process termed double fertilization. The resulting zygote develops into an embryo, while the triploid endosperm (one sperm cell plus two female cells) and female tissues of the ovule give rise to the surrounding tissues in the developing seed. The ovary, which produced the female gametophyte(s), then grows into a fruit, which surrounds the seed(s). Plants may either self-pollinate or cross-pollinate.
In 2013, flowers dating from the Cretaceous (100 million years before present) were found encased in amber, the oldest evidence of sexual reproduction in a flowering plant. Microscopic images showed tubes growing out of pollen and penetrating the flower's stigma. The pollen was sticky, suggesting it was carried by insects.[26]
Nonflowering plants like ferns, moss and liverworts use other means of sexual reproduction.
Ferns
Ferns mostly produce large diploid sporophytes with rhizomes, roots and leaves. Fertile leaves produce sporangia that contain haploid spores. The spores are released and germinate to produce short, thin gametophytes that are typically heart shaped, small and green in color. The gametophyte thalli, produce both motile sperm in the antheridia and egg cells in archegonia on the same or different plants. After rains or when dew deposits a film of water, the motile sperm are splashed away from the antheridia, which are normally produced on the top side of the thallus, and swim in the film of water to the archegonia where they fertilize the egg. To promote out crossing or cross fertilization the sperm are released before the eggs are receptive of the sperm, making it more likely that the sperm will fertilize the eggs of different thallus. After fertilization, a zygote is formed which grows into a new sporophytic plant. The condition of having separate sporophyte and gametophyte plants is called alternation of generations. Other plants with similar life cycles include Psilotum, Lycopodium, Selaginella and Equisetum.
Bryophytes
The bryophytes, which include liverworts, hornworts and mosses, reproduce both sexually and vegetatively. They are small plants found growing in moist locations and like ferns, have motile sperm with flagella and need water to facilitate sexual reproduction. These plants start as a haploid spore that grows into the dominant gametophyte form, which is a multicellular haploid body with leaf-like structures that photosynthesize. Haploid gametes are produced in antheridia (male) and archegonia (female) by mitosis. The sperm released from the antheridia respond to chemicals released by ripe archegonia and swim to them in a film of water and fertilize the egg cells thus producing a zygote. The zygote divides by mitotic division and grows into a multicellular, diploid sporophyte. The sporophyte produces spore capsules (sporangia), which are connected by stalks (setae) to the archegonia. The spore capsules produce spores by meiosis and when ripe the capsules burst open to release the spores. Bryophytes show considerable variation in their reproductive structures and the above is a basic outline. Also in some species each plant is one sex (dioicous) while other species produce both sexes on the same plant (monoicous).[27]
Fungi

Fungi are classified by the methods of sexual reproduction they employ. The outcome of sexual reproduction most often is the production of resting spores that are used to survive inclement times and to spread. There are typically three phases in the sexual reproduction of fungi: plasmogamy, karyogamy and meiosis. The cytoplasm of two parent cells fuse during plasmogamy and the nuclei fuse during karyogamy. New haploid gametes are formed during meiosis and develop into spores. The adaptive basis for the maintenance of sexual reproduction in the Ascomycota and Basidiomycota (dikaryon) fungi was reviewed by Wallen and Perlin.[28] They concluded that the most plausible reason for maintaining this capability is the benefit of repairing DNA damage, caused by a variety of stresses, through recombination that occurs during meiosis.[28]
Bacteria and archaea
Three distinct processes in prokaryotes are regarded as similar to eukaryotic sex: bacterial transformation, which involves the incorporation of foreign DNA into the bacterial chromosome; bacterial conjugation, which is a transfer of plasmid DNA between bacteria, but the plasmids are rarely incorporated into the bacterial chromosome; and gene transfer and genetic exchange in archaea.
Bacterial transformation involves the recombination of genetic material and its function is mainly associated with DNA repair. Bacterial transformation is a complex process encoded by numerous bacterial genes, and is a bacterial adaptation for DNA transfer.[12][13] This process occurs naturally in at least 40 bacterial species.[29] For a bacterium to bind, take up, and recombine exogenous DNA into its chromosome, it must enter a special physiological state referred to as competence (see Natural competence). Sexual reproduction in early single-celled eukaryotes may have evolved from bacterial transformation,[14] or from a similar process in archaea (see below).
On the other hand, bacterial conjugation is a type of direct transfer of DNA between two bacteria mediated by an external appendage called the conjugation pilus.[30] Bacterial conjugation is controlled by plasmid genes that are adapted for spreading copies of the plasmid between bacteria. The infrequent integration of a plasmid into a host bacterial chromosome, and the subsequent transfer of a part of the host chromosome to another cell do not appear to be bacterial adaptations.[12][31]
Exposure of hyperthermophilic archaeal Sulfolobus species to DNA damaging conditions induces cellular aggregation accompanied by high frequency genetic marker exchange.[32][33] Ajon et al.[33] hypothesized that this cellular aggregation enhances species-specific DNA repair by homologous recombination. DNA transfer in Sulfolobus may be an early form of sexual interaction similar to the more well-studied bacterial transformation systems that also involve species-specific DNA transfer leading to homologous recombinational repair of DNA damage.
See also
References
- John Maynard Smith & Eörz Szathmáry, The Major Transitions in Evolution, W. H. Freeman and Company, 1995, p 149
- "Fertilization". Merriam-Webster. Retrieved 2013-11-03.
- Otto, Sarah P.; Lenormand, Thomas (1 April 2002). "Resolving the paradox of sex and recombination". Nature Reviews Genetics. 3 (4): 252–261. doi:10.1038/nrg761. PMID 11967550.
- John Maynard Smith The Evolution of Sex 1978.
- Ridley M (2004) Evolution, 3rd edition. Blackwell Publishing, p. 314.
- Hussin, Julie G; Hodgkinson, Alan; Idaghdour, Youssef; Grenier, Jean-Christophe; Goulet, Jean-Philippe; Gbeha, Elias; Hip-Ki, Elodie; Awadalla, Philip (2015). "Recombination affects accumulation of damaging and disease-associated mutations in human populations". Nature Genetics. 47 (4): 400–404. doi:10.1038/ng.3216. PMID 25685891. Lay summary (4 March 2015).
- Cecie Starr (2013). Biology: The Unity & Diversity of Life (Ralph Taggart, Christine Evers, Lisa Starr ed.). Cengage Learning. p. 281.
- Vogt, Yngve (January 29, 2014). "Large testicles are linked to infidelity". Phys.org. Retrieved January 31, 2014.
- Agrawal, A. F. (2001). "Sexual selection and the maintenance of sexual reproduction". Nature. 411 (6838): 692–5. doi:10.1038/35079590. PMID 11395771.
- N.J. Buttefield (2000). "Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology. 26 (3): 386–404. doi:10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2.
- Gray, J. C.; Goddard, M. R. (2012). Bonsall, Michael (ed.). "Gene-flow between niches facilitates local adaptation in sexual populations". Ecology Letters. 15 (9): 955–962. doi:10.1111/j.1461-0248.2012.01814.x. PMID 22690742.
- Michod RE, Bernstein H, Nedelcu AM; Bernstein; Nedelcu (May 2008). "Adaptive value of sex in microbial pathogens" (PDF). Infect. Genet. Evol. 8 (3): 267–85. doi:10.1016/j.meegid.2008.01.002. PMID 18295550.CS1 maint: multiple names: authors list (link)
- Bernstein, Harris; Bernstein, Carol (2010). "Evolutionary Origin of Recombination during Meiosis". BioScience. 60 (7): 498–505. doi:10.1525/bio.2010.60.7.5.
- Bernstein H, Bernstein C, Michod RE. (2012) "DNA Repair as the Primary Adaptive Function of Sex in Bacteria and Eukaryotes". Chapter 1, pp. 1–50, in DNA Repair: New Research, Editors S. Kimura and Shimizu S. Nova Sci. Publ., Hauppauge, New York. Open access for reading only. ISBN 978-1-62100-756-2
- Colegrave, N (2002). "Sex releases the speed limit on evolution". Nature. 420 (6916): 664–6. Bibcode:2002Natur.420..664C. doi:10.1038/nature01191. hdl:1842/692. PMID 12478292.
- Kleiman, Maya; Tannenbaum, Emmanuel (2009). "Diploidy and the selective advantage for sexual reproduction in unicellular organisms". Theory in Biosciences. 128 (4): 249–85. arXiv:0901.1320. doi:10.1007/s12064-009-0077-9. PMID 19902285.
- Dimijian, G. G. (2005). Evolution of sexuality: biology and behavior. Proceedings (Baylor University. Medical Center), 18, 244–258.
- Hamilton, W.D. (1967). "Extraordinary sex ratios". Science. 156 (3774): 477–488. Bibcode:1967Sci...156..477H. doi:10.1126/science.156.3774.477. PMID 6021675.
- Reichard, U.H. (2002). "Monogamy—A variable relationship" (PDF). Max Planck Research. 3: 62–7. Archived from the original (PDF) on 24 May 2013. Retrieved 24 April 2013.
- Lipton, Judith Eve; Barash, David P. (2001). The Myth of Monogamy: Fidelity and Infidelity in Animals and People. San Francisco: W.H. Freeman and Company. ISBN 0-7167-4004-4.
- Research conducted by Patricia Adair Gowaty. Reported by Morell, V. (1998). "Evolution of sex: A new look at monogamy". Science. 281 (5385): 1982–1983. doi:10.1126/science.281.5385.1982. PMID 9767050.
- BONY FISHES - Reproduction
- M. Cavendish (2001). Endangered Wildlife and Plants of the World. Marshall Cavendish. p. 1252. ISBN 978-0-7614-7194-3. Retrieved 2013-11-03.
- Orlando EF, Katsu Y, Miyagawa S, Iguchi T; Katsu; Miyagawa; Iguchi (2006). "Cloning and differential expression of estrogen receptor and aromatase genes in the self-fertilizing hermaphrodite and male mangrove rivulus, Kryptolebias marmoratus". Journal of Molecular Endocrinology. 37 (2): 353–365. doi:10.1677/jme.1.02101. PMID 17032750.CS1 maint: multiple names: authors list (link)
- I. Schlupp, J. Parzefall, J. T. Epplen, M. Schartl; Parzefall; Epplen; Schartl (2006). "Limia vittata as host species for the Amazon molly: no evidence for sexual reproduction". Journal of Fish Biology. 48 (4): 792–795. doi:10.1111/j.1095-8649.1996.tb01472.x.CS1 maint: multiple names: authors list (link)
- Poinar Jr., George O; Chambers, Kenton L; Wunderlich, Joerg (10 December 2013). "Micropetasos, a new genus of angiosperms from mid-Cretaceous Burmese amber". J. Bot. Res. Inst. Texas. 7 (2): 745–750. Archived from the original (PDF) on 5 January 2014. Lay summary (3 January 2014).
- Jon Lovett Doust; Lesley Lovett Doust (1988). Plant Reproductive Ecology: Patterns and Strategies. Oxford University Press. p. 290. ISBN 9780195063943.
- Wallen RM, Perlin MH (2018). "An Overview of the Function and Maintenance of Sexual Reproduction in Dikaryotic Fungi". Front Microbiol. 9: 503. doi:10.3389/fmicb.2018.00503. PMC 5871698. PMID 29619017.
- Lorenz, MG; Wackernagel, W (1994). "Bacterial gene transfer by natural genetic transformation in the environment". Microbiological Reviews. 58 (3): 563–602. doi:10.1128/mmbr.58.3.563-602.1994. PMC 372978. PMID 7968924.
- Lodé, T (2012). "Have Sex or Not? Lessons from Bacteria". Sexual Development : Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation. 6 (6): 325–8. doi:10.1159/000342879. PMID 22986519.
- Krebs, JE; Goldstein, ES; Kilpatrick, ST (2011). Lewin's GENES X. Boston: Jones and Bartlett Publishers. pp. 289–292. ISBN 9780763766320.
- Fröls S, Ajon M, Wagner M, Teichmann D, Zolghadr B, Folea M, Boekema EJ, Driessen AJ, Schleper C, Albers SV (2008). "UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation" (PDF). Mol Microbiol. 70 (4): 938–952. doi:10.1111/j.1365-2958.2008.06459.x. PMID 18990182.
- Ajon M, Fröls S, van Wolferen M, Stoecker K, Teichmann D, Driessen AJ, Grogan DW, Albers SV, Schleper C; Fröls; Van Wolferen; Stoecker; Teichmann; Driessen; Grogan; Albers; Schleper (November 2011). "UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili" (PDF). Mol. Microbiol. 82 (4): 807–17. doi:10.1111/j.1365-2958.2011.07861.x. PMID 21999488.CS1 maint: multiple names: authors list (link)
Further reading
- Pang, K. "Certificate Biology: New Mastering Basic Concepts", Hong Kong, 2004
- Journal of Biology of Reproduction, accessed in August 2005.
- "Sperm Use Heat Sensors To Find The Egg; Weizmann Institute Research Contributes To Understanding Of Human Fertilization", Science Daily, 3 February 2003
- Michod, RE; Levin, BE, eds. (1987). The Evolution of sex: An examination of current ideas. Sunderland, Massachusetts: Sinauer Associates. ISBN 978-0878934584.
- Michod, RE (1994). Eros and Evolution: A Natural Philosophy of Sex. Perseus Books. ISBN 978-0201407549.
