Nandrolone
Nandrolone, also known as 19-nortestosterone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as nandrolone decanoate (brand name Deca-Durabolin) and nandrolone phenylpropionate (brand name Durabolin).[2][11][8][12] Nandrolone esters are used in the treatment of anemias, cachexia (wasting syndrome), osteoporosis, breast cancer, and for other indications.[8] They are not used by mouth and instead are given by injection into muscle or fat.[8][12][13]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈnændrəloʊn/[1] |
| Trade names | • Deca-Durabolin (as ND) • Durabolin (as NPP) • Many others (see here) |
| Other names | • 19-Nortestosterone[2][3] • 10-Nortestosterone • Estr-4-en-17β-ol-3-one • Estrenolone • Oestrenolone • 19-Norandrost-4-en-17β-ol-3-one • Norandrostenolone[2] • Nortestrionate[2] • Nortestonate[2] • SG-4341[2][3] |
| Pregnancy category | |
| Routes of administration | • IM injection (esters) • SC injection (esters) • Eye drops (NS) |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | • Oral: <3% (pigs)[4] • Intramuscular: high[5] |
| Metabolism | Liver (reduction)[6][7] |
| Metabolites | • 5α-Dihydronandrolone[6][8] • 19-Norandrosterone[6] • 19-Noretiocholanolone[6] • Conjugates[7] |
| Elimination half-life | • Nandrolone: <4.3 hours[6] • ND (IM): 6–12 days[6][8][9] • NPP: 2.7 days[9] |
| Duration of action | • ND (IM): 2–3 weeks[8][10] • NPP (IM): 5–7 days[8][9] |
| Excretion | Urine[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.457 |
| Chemical and physical data | |
| Formula | C18H26O2 |
| Molar mass | 274.404 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects of nandrolone esters include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[8] They are synthetic androgens and anabolic steroids and hence are agonists of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[8][14] Nandrolone esters have strong anabolic effects and weak androgenic effects, which give them a mild side effect profile and make them especially suitable for use in women and children.[8][14][15] They are long-lasting prodrugs of nandrolone in the body.[8]
Nandrolone esters were first described and introduced for medical use in the late 1950s.[8] They are among the most widely used AAS worldwide.[8] In addition to their medical use, nandrolone esters are used to improve physique and performance, and are said to be the most widely used AAS for such purposes.[8][16] The drugs are controlled substances in many countries and so non-medical use is generally illicit.[8]
Medical uses
Nandrolone esters are used clinically, although increasingly rarely, for people in catabolic states with major burns, cancer, and AIDS, and an ophthalmological formulation was available to support cornea healing.[17]:134
The positive effects of nandrolone esters include muscle growth, appetite stimulation and increased red blood cell production, and bone density.[18] Clinical studies have shown them to be effective in treating anemia, osteoporosis, and breast cancer.
Nandrolone sulfate has been used in an eye drop formulation as an ophthalmic medication.[2][11]
Non-medical uses
Nandrolone esters are used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters.[8]
Side effects
Side effects of nandrolone esters include masculinization among others.[8] In women, nandrolone and nandrolone esters have been reported to produce increased libido, acne, facial and body hair growth, voice changes, and clitoral enlargement.[19] However, the masculinizing effects of nandrolone and its esters are reported to be slighter than those of testosterone.[19] Nandrolone has also been found to produce penile growth in prepubertal boys.[19] Amenorrhea and menorrhagia have been reported as side effects of nandrolone cypionate.[19]
Nandrolone theoretically may produce erectile dysfunction as a side effect, although there is no clinical evidence to support this notion at present.[20] Side effects of high doses of nandrolone may include cardiovascular toxicity as well as hypogonadism and infertility. Nandrolone may not produce scalp hair loss, although this is also theoretical.[20]
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Nandrolone is an agonist of the AR, the biological target of androgens like testosterone and DHT. Unlike testosterone and certain other AAS, nandrolone is not potentiated in androgenic tissues like the scalp, skin, and prostate, hence deleterious effects in these tissues are lessened.[21] This is because nandrolone is metabolized by 5α-reductase to the much weaker AR ligand 5α-dihydronandrolone (DHN), which has both reduced affinity for the androgen receptor (AR) relative to nandrolone in vitro and weaker AR agonistic potency in vivo.[21] The lack of alkylation on the 17α-carbon drastically reduces the hepatotoxic potential of nandrolone. Estrogen effects resulting from reaction with aromatase are also reduced due to lessened enzyme interaction,[22] but effects such as gynecomastia and reduced libido may still occur at sufficiently high doses.
In addition to its AR agonistic activity, unlike many other AAS, nandrolone is also a potent progestogen.[23] It binds to the progesterone receptor with approximately 22% of the affinity of progesterone.[23] The progestogenic activity of nandrolone serves to augment its antigonadotropic effects,[24][8] as antigonadotropic action is a known property of progestogens.[25][26]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Nandrolone | 20 | 154–155 | <0.1 | 0.5 | 1.6 | 1–16 | 0.1 |
| Testosterone | 1.0–1.2 | 100 | <0.1 | 0.17 | 0.9 | 19–82 | 3–8 |
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Sources: See template. | |||||||
Anabolic and androgenic activity
Nandrolone has a very high ratio of anabolic to androgenic activity.[14] In fact, nandrolone-like AAS like nandrolone itself and trenbolone are said to have among the highest ratio of anabolic to androgenic effect of all AAS.[24] This is attributed to the fact that whereas testosterone is potentiated via conversion into dihydrotestosterone (DHT) in androgenic tissues, the opposite is true with nandrolone and similar AAS (i.e., other 19-nortestosterone derivatives).[14] As such, nandrolone-like AAS, namely nandrolone esters, are the most frequently used AAS in clinical settings in which anabolic effects are desired; for instance, in the treatment of AIDS-associated cachexia, severe burns, and chronic obstructive pulmonary disease.[24] However, AAS with a very high ratio of anabolic to androgenic action like nandrolone still have significant androgenic effects and can produce symptoms of masculinization like hirsutism and voice deepening in women and children with extended use.[14]
| Compound | rAR (%) | hAR (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Testosterone | 38 | 38 | ||||||
| 5α-Dihydrotestosterone | 77 | 100 | ||||||
| Nandrolone | 75 | 92 | ||||||
| 5α-Dihydronandrolone | 35 | 50 | ||||||
| Ethylestrenol | ND | 2 | ||||||
| Norethandrolone | ND | 22 | ||||||
| 5α-Dihydronorethandrolone | ND | 14 | ||||||
| Metribolone | 100 | 110 | ||||||
| Sources: See template. | ||||||||
Pharmacokinetics
The oral activity of nandrolone has been studied.[27][28][29][30][31][32] With oral administration of nandrolone in rodents, it had about one-tenth of the potency of subcutaneous injection of nandrolone.[27][33][19]
Nandrolone has very low affinity for human serum sex hormone-binding globulin (SHBG), about 5% of that of testosterone and 1% of that of DHT.[34] It is metabolized by the enzyme 5α-reductase, among others.[35] Nandrolone is less susceptible to metabolism by 5α-reductase and 17β-hydroxysteroid dehydrogenase than testosterone.[35] This results in it being transformed less in so-called "androgenic" tissues like the skin, hair follicles, and prostate gland and in the kidneys, respectively.[35] Metabolites of nandrolone include 5α-dihydronandrolone, 19-norandrosterone, and 19-noretiocholanolone, and these metabolites may be detected in urine.[36]
Single intramuscular injections of 100 mg nandrolone phenylpropionate or nandrolone decanoate have been found to produce an anabolic effect for 10 to 14 days and 20 to 25 days, respectively.[37] Conversely, unesterified nandrolone has been used by intramuscular injection once daily.[19][33]
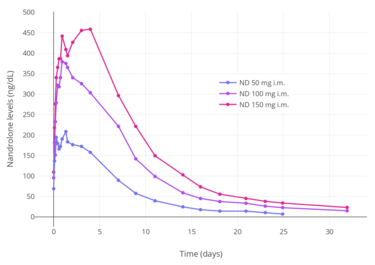 Nandrolone levels after a single 50, 100, or 150 mg intramuscular injection of nandrolone decanoate in oil solution in men.[38]
Nandrolone levels after a single 50, 100, or 150 mg intramuscular injection of nandrolone decanoate in oil solution in men.[38]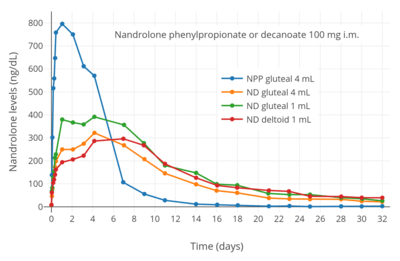 Nandrolone levels after a single 100 mg intramuscular injection of nandrolone decanoate or nandrolone phenylpropionate in 4 mL or 1 mL arachis oil solution into gluteal or deltoid muscle in men.[9]
Nandrolone levels after a single 100 mg intramuscular injection of nandrolone decanoate or nandrolone phenylpropionate in 4 mL or 1 mL arachis oil solution into gluteal or deltoid muscle in men.[9]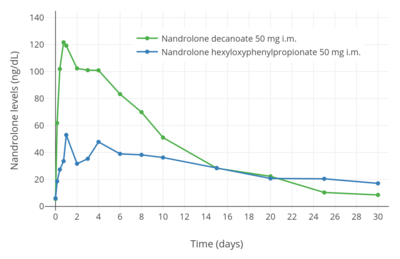 Nandrolone levels with a single 50 mg intramuscular injection of nandrolone decanoate or nandrolone hexyloxyphenylpropionate in oil solution in men.[39]
Nandrolone levels with a single 50 mg intramuscular injection of nandrolone decanoate or nandrolone hexyloxyphenylpropionate in oil solution in men.[39]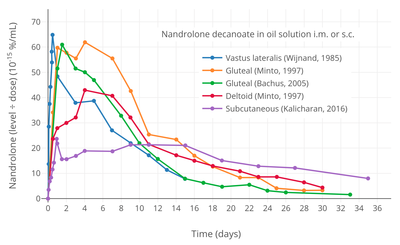 Dose-normalized nandrolone exposure (serum level divided by dose administered) with nandrolone decanoate in oil solution by intramuscular or subcutaneous injection in men.[40][41]
Dose-normalized nandrolone exposure (serum level divided by dose administered) with nandrolone decanoate in oil solution by intramuscular or subcutaneous injection in men.[40][41]
Chemistry
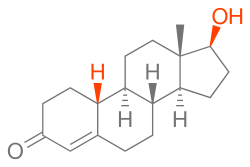
Nandrolone, also known as 19-nortestosterone (19-NT) or as estrenolone, as well as estra-4-en-17β-ol-3-one or 19-norandrost-4-en-17β-ol-3-one,[42] is a naturally occurring estrane (19-norandrostane) steroid and a derivative of testosterone (androst-4-en-17β-ol-3-one).[2][11] It is specifically the C19 demethylated (nor) analogue of testosterone.[2][11] Nandrolone is an endogenous intermediate in the production of estradiol from testosterone via aromatase in mammals including humans and is present in the body naturally in trace amounts.[43] It can be detected during pregnancy in women.[44] Nandrolone esters have an ester such as decanoate or phenylpropionate attached at the C17β position.[2][11]
Derivatives
Esters
A variety of esters of nandrolone have been marketed and used medically.[2][11] The most commonly used esters are nandrolone decanoate and to a lesser extent nandrolone phenylpropionate. Examples of other nandrolone esters that have been marketed and used medically include nandrolone cyclohexylpropionate, nandrolone cypionate, nandrolone hexyloxyphenylpropionate, nandrolone laurate, nandrolone sulfate, and nandrolone undecanoate.[2][11][8]
Anabolic steroids
Nandrolone is the parent compound of a large group of AAS. Notable examples include the non-17α-alkylated trenbolone and the 17α-alkylated ethylestrenol (ethylnandrol) and metribolone (R-1881), as well as the 17α-alkylated designer steroids norboletone and tetrahydrogestrinone (THG). The following is list of derivatives of nandrolone that have been developed as AAS:[8]
|
|
Progestins
Nandrolone, together with ethisterone (17α-ethynyltestosterone), is also the parent compound of a large group of progestins, the norethisterone (17α-ethynyl-19-nortestosterone) derivatives.[45][46] This family is subdivided into two groups: the estranes and the gonanes.[45] The estranes include norethisterone (norethindrone), norethisterone acetate, norethisterone enanthate, lynestrenol, etynodiol diacetate, and noretynodrel, while the gonanes include norgestrel, levonorgestrel, desogestrel, etonogestrel, gestodene, norgestimate, dienogest (actually a 17α-cyanomethyl-19-nortestosterone derivative), and norelgestromin.[45]
Synthesis
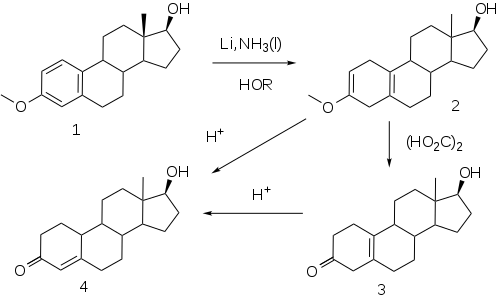
The elaboration of a method for the reduction of aromatic rings to the corresponding dihydrobenzenes under controlled conditions by A. J. Birch opened a convenient route to compounds related to the putative 19-norprogesterone.
This reaction, now known as the Birch reduction,[50] is typified by the treatment of the monomethyl ether of estradiol (1) with a solution of lithium metal in liquid ammonia in the presence of alcohol as a proton source. Initial reaction constituents of 1,4-dimetalation of the most electron deficient positions of the aromatic ring–in the case of an estrogen, the 1 and 4-positions. Rxn of the intermediate with the proton source leads to a dihydrobenzene; a special virtue of this sequence in steroids is the fact that the double bind at 2 is in effect becomes an enol ether moiety. Treatment of this product (2) with weak acid, oxalic acid for e.g., leads to the hydrolysis of the enol ether, producing β,γ-unconjugated ketone 3. Hydrolysis under more strenuous conditions (mineral acids) results in migration/conjugation of the olefin to yield nandrolone (4).
Esters
Detection in body fluids
Nandrolone use is directly detectable in hair or indirectly detectable in urine by testing for the presence of 19-norandrosterone, a metabolite. The International Olympic Committee has set a limit of 2.0 μg/L of 19-norandrosterone in urine as the upper limit,[53] beyond which an athlete is suspected of doping. In the largest nandrolone study performed on 621 athletes at the 1998 Nagano Olympic Games, no athlete tested over 0.4 μg/L. 19-Norandrosterone was identified as a trace contaminant in commercial preparations of androstenedione, which until 2004 was available without a prescription as a dietary supplement in the U.S.[54][55][56][57]
A number of nandrolone cases in athletics occurred in 1999, which included high-profile athletes such as Merlene Ottey, Dieter Baumann and Linford Christie.[58] However, the following year the detection method for nandrolone at the time was proved to be faulty. Mark Richardson, a British Olympic relay runner who tested positive for the substance, gave a significant amount of urine samples in a controlled environment and delivered a positive test for the drug, demonstrating that false positives could occur, which led to an overhaul of his competitive ban.[59]
Heavy consumption of the essential amino acid lysine (as indicated in the treatment of cold sores) has allegedly shown false positives in some and was cited by American shotputter C. J. Hunter as the reason for his positive test, though in 2004 he admitted to a federal grand jury that he had injected nandrolone.[60] A possible cause of incorrect urine test results is the presence of metabolites from other AAS, though modern urinalysis can usually determine the exact AAS used by analyzing the ratio of the two remaining nandrolone metabolites. As a result of the numerous overturned verdicts, the testing procedure was reviewed by UK Sport. On October 5, 2007, three-time Olympic gold medalist for track and field Marion Jones admitted to use of the drug, and was sentenced to six months in jail for lying to a federal grand jury in 2000.[61]
Mass spectrometry is also used to detect small samples of nandrolone in urine samples, as it has a unique molar mass.
History

Nandrolone was first synthesized in 1950.[2][42][17]:130[62] It was first introduced, as nandrolone phenylpropionate, in 1959, and then as nandrolone decanoate in 1962, followed by additional esters.[63]
Society and culture
Generic names
Nandrolone is the generic name of the drug and its INN, BAN, DCF, and DCIT.[2][11][3][64] The formal generic names of nandrolone esters include nandrolone cyclohexylpropionate (BANM), nandrolone cyclotate (USAN), nandrolone decanoate (USAN, USP, BANM, JAN), nandrolone laurate (BANM), nandrolone phenpropionate (USP), and nandrolone phenylpropionate (BANM, JAN).[2][11][3][64]
Doping in sports
Nandrolone was probably among the first AAS to be used as a doping agent in sports in the 1960s. It has been banned at the Olympics since 1974.[17]:128 There are many known cases of doping in sports with nandrolone esters by professional athletes.
Research
Nandrolone esters have been studied in several indications. They were intensively studied for osteoporosis, and increased calcium uptake and decreased bone loss, but caused virilization in about half of the women who took them and were mostly abandoned for this use when better drugs like the bisphosphonates became available.[20] They have also been studied in clinical trials for chronic kidney failure, aplastic anemia, and as male contraceptives.[17]:134
References
- "Nandrolone Meaning in the Cambridge English Dictionary".
- Elks, J; Ganellin, C R, eds. (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. New York City: Springer. pp. 660–. ISBN 978-1-4757-2085-3. OCLC 1079003025.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- McEvoy JD, McVeigh CE, McCaughey WJ (1998). "Residues of nortestosterone esters at injection sites. Part 1. Oral bioavailability". Analyst. 123 (12): 2475–8. doi:10.1039/a804919j. PMID 10435281.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1185–. ISBN 978-0-7817-1750-2.
- http://www.medsafe.govt.nz/profs/Datasheet/d/Decadurabolininj.pdf
- John A. Thomas (6 December 2012). Drugs, Athletes, and Physical Performance. Springer Science & Business Media. pp. 27–29. ISBN 978-1-4684-5499-4.
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 402–412, 460–467, 193–194. ISBN 978-0-9828280-1-4.
- Minto CF, Howe C, Wishart S, Conway AJ, Handelsman DJ (1997). "Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and injection volume". J. Pharmacol. Exp. Ther. 281 (1): 93–102. PMID 9103484.
- https://gp2u.com.au/static/pdf/D/DECA-DURABOLIN-PI.pdf
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 716–. ISBN 978-3-88763-075-1.
- Walter Sneader (23 June 2005). Drug Discovery: A History. John Wiley & Sons. pp. 206–. ISBN 978-0-471-89979-2.
- Singh, Gurmeet K. S.; Turner, Leo; Desai, Reena; Jimenez, Mark; Handelsman, David J. (2014). "Pharmacokinetic-Pharmacodynamic Study of Subcutaneous Injection of Depot Nandrolone Decanoate Using Dried Blood Spots Sampling Coupled With Ultrapressure Liquid Chromatography Tandem Mass Spectrometry Assays". The Journal of Clinical Endocrinology & Metabolism. 99 (7): 2592–2598. doi:10.1210/jc.2014-1243. ISSN 0021-972X. PMID 24684468.
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 401–. ISBN 978-3-642-66353-6.
- J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2388–. ISBN 978-0-323-32195-2.
- Hemmersbach, Peter; Große, Joachim (2009). "Nandrolone: A Multi-Faceted Doping Agent". In Thieme, Detlef; Hemmersbach, Peter (eds.). Doping in sports. Berlin: Springer. pp. 127–154. ISBN 978-3-540-79088-4.
- Handelsman, David J (2013). "Androgen Physiology, Pharmacology and Abuse". In De Groot, Leslie J (ed.). Endotext. 4.1.2 Pharmacologic Androgen Therapy – via NCBI Bookshelf.
Both testosterone and its non-aromatizable derivative nandrolone, produce increased bone density in men with glucocorticoid-induced osteoporosis with minimal short-term side-effects....
- Camerino, B.; Sala, G. (1960). "Anabolic Steroids". Fortschritte der Arzneimittelforschung / Progress in Drug Research / Progrès des recherches pharmaceutiques. Fortschritte der Arzneimittelforschung. Progress in Drug Research. Progres des Recherches Pharmaceutiques. 2. pp. 71–134. doi:10.1007/978-3-0348-7038-2_2. ISBN 978-3-0348-7040-5. PMID 14448579.
- Pan, MM; Kovac, JR (April 2016). "Beyond testosterone cypionate: evidence behind the use of nandrolone in male health and wellness". Translational Andrology and Urology. 5 (2): 213–9. doi:10.21037/tau.2016.03.03. PMC 4837307. PMID 27141449.
- Bergink EW, Janssen PS, Turpijn EW, van der Vies J (June 1985). "Comparison of the receptor binding properties of nandrolone and testosterone under in vitro and in vivo conditions". J. Steroid Biochem. 22 (6): 831–6. doi:10.1016/0022-4731(85)90293-6. PMID 4021486.
- Brueggemeier RW (September 16, 2006). "Sex Hormones (Male): Analogs and Antagonists". In Meyers RA (ed.). Encyclopedia of Molecular Cell Biology and Molecular Medicine. Encyclopedia of Molecular Cell Biology and Molecular Medicine (abstract). John Wiley & Sons. doi:10.1002/3527600906.mcb.200500066. ISBN 978-3527600908.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- de Souza GL, Hallak J (2011). "Anabolic steroids and male infertility: a comprehensive review". BJU Int. 108 (11): 1860–5. doi:10.1111/j.1464-410X.2011.10131.x. PMID 21682835.
- Couzinet B, Young J, Brailly S, Chanson P, Thomas JL, Schaison G (1996). "The antigonadotropic activity of progestins (19-nortestosterone and 19-norprogesterone derivatives) is not mediated through the androgen receptor". J. Clin. Endocrinol. Metab. 81 (12): 4218–23. doi:10.1210/jcem.81.12.8954018. PMID 8954018.
- Mauvais-Jarvis, P. "Progesterone and progestins: a general overview." (1983): 1-16.
- Camerino, B.; Sciaky, R. (1975). "Structure and effects of anabolic steroids". Pharmacology & Therapeutics. Part B: General and Systematic Pharmacology. 1 (2): 233–275. doi:10.1016/0306-039X(75)90007-0. ISSN 0306-039X. PMID 817322.
- Holtkamp DE, Heming AE, Mansor LF (1955). "Comparison of Oral and Subcutaneous Administration in the Anabolic and Androgenic Effectiveness of 19-Nortestosterone and Testosterone". The Journal of Clinical Endocrinology & Metabolism. 15 (7): 848. doi:10.1210/jcem-15-7-834. ISSN 0021-972X.
- Furman RH, Howard RP, Smith CW, Norcia LN (January 1956). "Comparison of the effects of oral methyltestosterone, 19-nortestosterone, and 17-methyl-19-nortestosterone on serum lipids and lipoproteins". Journal of Laboratory and Clinical Medicine. 48 (5): 808–809.
- Furman, Robert H.; Howard, R.Palmer; Norcia, Leonard N.; Keaty, E.Corinne (1958). "The influence of androgens, estrogens and related steroids on serum lipids and lipoproteins". The American Journal of Medicine. 24 (1): 80–97. doi:10.1016/0002-9343(58)90364-4. ISSN 0002-9343. PMID 13498038.
- McEvoy, John D. G.; McVeigh, Cynthia E.; John McCaughey, W. (1998). "Residues of nortestosterone esters at injection sites. Part 1. Oral bioavailability". The Analyst. 123 (12): 2475–2478. doi:10.1039/a804919j. ISSN 0003-2654. PMID 10435281.
- Arnold A, Potts GO (January 1964). "Oral anabolic and androgenic effects of 19-nortestosterone and 17α-methyl-19-nortestosterone". Federation Proceedings. 23 (2): 412.
- Baker WH, Henneman PH, Baggett B, Engel LL, Tibbetts D, Brown M (January 1955). "Metabolic effect of 19-nortestosterone". Journal of Clinical Endocrinology & Metabolism. 15 (7): 848–849. doi:10.1210/jcem-15-7-834. ISSN 0021-972X.
- Saartok T, Dahlberg E, Gustafsson JA (June 1984). "Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin". Endocrinology. 114 (6): 2100–6. doi:10.1210/endo-114-6-2100. PMID 6539197.
- Bergink EW, Geelen JA, Turpijn EW (1985). "Metabolism and receptor binding of nandrolone and testosterone under in vitro and in vivo conditions". Acta Endocrinol Suppl (Copenh). 271 (3_Suppla): 31–7. doi:10.1530/acta.0.109S0031. PMID 3865479.
- David R. Mottram (12 November 2010). Drugs in Sport. Routledge. pp. 63–. ISBN 978-1-135-25825-2.
- Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 68–. ISBN 978-1-4832-7300-6.
- Bagchus WM, Smeets JM, Verheul HA, De Jager-Van Der Veen SM, Port A, Geurts TB (2005). "Pharmacokinetic evaluation of three different intramuscular doses of nandrolone decanoate: analysis of serum and urine samples in healthy men". J. Clin. Endocrinol. Metab. 90 (5): 2624–30. doi:10.1210/jc.2004-1526. PMID 15713722.
- Belkien L, Schürmeyer T, Hano R, Gunnarsson PO, Nieschlag E (May 1985). "Pharmacokinetics of 19-nortestosterone esters in normal men". J. Steroid Biochem. 22 (5): 623–9. doi:10.1016/0022-4731(85)90215-8. PMID 4010287.
- Kalicharan RW, Schot P, Vromans H (February 2016). "Fundamental understanding of drug absorption from a parenteral oil depot". Eur J Pharm Sci. 83: 19–27. doi:10.1016/j.ejps.2015.12.011. PMID 26690043.
- Kalicharan, Raween Wikesh (2017). New Insights into Drug Absorption from Oil Depots (Thesis). Utrecht University.
- R Schnitzer (1 January 1967). Experimental Chemotherapy. Elsevier Science. pp. 165–. ISBN 978-0-323-14611-1.
- Bricout V, Wright F (2004). "Update on nandrolone and norsteroids: how endogenous or xenobiotic are these substances?". Eur. J. Appl. Physiol. 92 (1–2): 1–12. doi:10.1007/s00421-004-1051-3. PMID 15042372.
- Lippi G, Franchini M, Banfi G (2011). "Biochemistry and physiology of anabolic androgenic steroids doping". Mini Rev Med Chem. 11 (5): 362–73. doi:10.2174/138955711795445952. PMID 21443514.
- Schindler, Adolf E; Campagnoli, Carlo; Druckmann, René; Huber, Johannes; Pasqualini, Jorge R; Schweppe, Karl W; Thijssen, Jos H.H (2003). "Classification and pharmacology of progestins". Maturitas. 46: 7–16. doi:10.1016/j.maturitas.2003.09.014. ISSN 0378-5122. PMID 14670641.
- A. Wayne Meikle (24 April 2003). Endocrine Replacement Therapy in Clinical Practice. Springer Science & Business Media. pp. 489–. ISBN 978-1-59259-375-0.
Estranes. Estrane and gonane progestogens are derived from 19-nortestosterone, the progestogenic parent compound used in oral contraceptives in the United States. Estranes are characterized by the presence of an ethinyl group at position 17 and by the absence of a methyl group between the A and B rings (see Fig. 10). The estrane progestogens that are related structurally to norethindrone (norethynodrel, lynestrenol, norethindrone acetate, ethynodiol diacetate) are converted to this parent compound. Norethindrone is the second most commonly used progestogen in the United States for HRT. Gonanes. The gonanes share the structural modifications found in the estranes and also possess an ethinyl group at position 13 and a keto group at position 3 (see Fig. 11). Norgestrel was synthesized in 1963 and is a racemic mixture of dextro and levorotatory forms. The levorotatory form, levonorgestrel, provides the biologic activity. Third-generation gonanes (desogestrel, gestodene, and norgestimate) have been developed to reduce unwanted side effects of progestogens, [...]
- Wilds, A. L.; Nelson, Norman A. (1953). "The Facile Synthesis of 19-Nortestosterone and 19-Norandrostenedione from Estrone". Journal of the American Chemical Society. 75 (21): 5366–5369. doi:10.1021/ja01117a065.
- Ueberwasser, H.; Heusler, K.; Kalvoda, J.; Meystre, Ch.; Wieland, P.; Anner, G.; Wettstein, A. (1963). "19-Norsteroide II. Ein einfaches Herstellungsverfahren für 19-Norandrostan-Derivate. Über Steroide, 193. Mitteilung". Helvetica Chimica Acta. 46: 344–352. doi:10.1002/hlca.19630460135.
- Shimizu, Isao; Naito, Yoichiro; Tsuji, Jiro (1980). "Synthesis of optically active (+)-19-nortestosterone by asymmetric bis-annulation reaction". Tetrahedron Letters. 21 (5): 487–490. doi:10.1016/S0040-4039(00)71440-7.
- Birch, Arthur J. (1950). "The reduction of organic compounds by metal-ammonia solutions". Quarterly Reviews, Chemical Society. 4: 69. doi:10.1039/QR9500400069.
- DeWytt, E. D.; Overbeek, O.; Overbeek, G. A.; U.S. Patent 2,998,423 (1961 to Organon).
- CH 206119 (1939 to Gesellschaft für Chemische Industrie Basel).
- "Clarification about Nandrolone Testing". World Anti-Doping Agency. 2005-05-30. Archived from the original on 2012-09-15. Retrieved 2012-01-31.
- Bresson M, Cirimele V, Villain M, Kintz P (May 2006). "Doping control for metandienone using hair analyzed by gas chromatography-tandem mass spectrometry". J. Chromatogr. B. 836 (1–2): 124–8. doi:10.1016/j.jchromb.2006.03.040. PMID 16597518.
- Ueki M, Ikekita A, Takao Y (2000). "[Nandrolone metabolite in urine of Nagano Olympic athlete]". Jap. J. For. Tox. (in Japanese). 18: 198–199.
- Catlin DH, Leder BZ, Ahrens B, Starcevic B, Hatton CK, Green GA, Finkelstein JS (2000). "Trace contamination of over-the-counter androstenedione and positive urine test results for a nandrolone metabolite". JAMA. 284 (20): 2618–21. doi:10.1001/jama.284.20.2618. PMID 11086369.
- Baselt RC (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 1078–1080. ISBN 978-0-9626523-7-0.
- Baron, Peter (2000-09-19). The Drugs Issue: Baumann to fight all the way. The Daily Telegraph. Retrieved on 2010-11-13.
- Richardson M (2004-02-19). "Athletics: Mark Richardson explains how he felt at his disciplinary hearing". Athletics. London: The Guardian. Retrieved 2010-11-13.
It was as daunting a line-up as I had ever faced on athletics tracks
- "Track Star Marion Jones to Admit Steroid Use". National Public Radio. 2007-10-05. Retrieved 2009-11-09.
- UK Sport Anti-doping Directorate (January 2000). Nandrolone Review (PDF) (Report). UK Sport. Archived from the original (PDF) on 2005-04-04. Retrieved 2013-02-02.
- Birch, Arthur J. (1950). "80. Hydroaromatic steroid hormones. Part I. 10-Nortestosterone". Journal of the Chemical Society (Resumed): 367. doi:10.1039/jr9500000367. ISSN 0368-1769.
- Consolidated List of Products Whose Consumption And/or Sale Have Been Banned, Withdrawn, Severely Restricted Or Not Approved by Governments. United Nations Publications. 1983. pp. 154–. ISBN 978-92-1-130230-1.
- "Nandrolone".
Further reading
- Geusens P (1995). "Nandrolone decanoate: pharmacological properties and therapeutic use in osteoporosis". Clin. Rheumatol. 14 Suppl 3: 32–9. doi:10.1007/bf02210686. PMID 8846659.
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Hemmersbach P, Grosse J (2010). Nandrolone: a multi-faceted doping agent. Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 195. pp. 127–54. doi:10.1007/978-3-540-79088-4_6. ISBN 978-3-540-79087-7. PMID 20020363.
- Velema MS, Kwa BH, de Ronde W (2012). "Should androgenic anabolic steroids be considered in the treatment regime of selected chronic obstructive pulmonary disease patients?". Curr Opin Pulm Med. 18 (2): 118–24. doi:10.1097/MCP.0b013e32834e9001. PMID 22189453.
- Busardò FP, Frati P, Sanzo MD, Napoletano S, Pinchi E, Zaami S, Fineschi V (2015). "The impact of nandrolone decanoate on the central nervous system". Curr Neuropharmacol. 13 (1): 122–31. doi:10.2174/1570159X13666141210225822. PMC 4462037. PMID 26074747.
- Wu C, Kovac JR (2016). "Novel Uses for the Anabolic Androgenic Steroids Nandrolone and Oxandrolone in the Management of Male Health". Curr Urol Rep. 17 (10): 72. doi:10.1007/s11934-016-0629-8. PMID 27535042.
- Pan MM, Kovac JR (2016). "Beyond testosterone cypionate: evidence behind the use of nandrolone in male health and wellness". Transl Androl Urol. 5 (2): 213–9. doi:10.21037/tau.2016.03.03. PMC 4837307. PMID 27141449.