Acetaldehyde
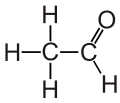
Acetaldehyde (systematic name ethanal) is an organic chemical compound with the formula CH3CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit,[10] and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke.[11] Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acetaldehyde[1] | |||
| Systematic IUPAC name
Ethanal[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.761 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4O | |||
| Molar mass | 44.053 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Ethereal | ||
| Density | 0.784 g·cm−3 (20 °C) [4]
0.7904–0.7928 g·cm−3 (10 °C)[4] | ||
| Melting point | −123.37 °C (−190.07 °F; 149.78 K) | ||
| Boiling point | 20.2 °C (68.4 °F; 293.3 K) | ||
| miscible | |||
| Solubility | miscible with ethanol, ether, benzene, toluene, xylene, turpentine, acetone slightly soluble in chloroform | ||
| log P | -0.34 | ||
| Vapor pressure | 740 mmHg (20 °C)[5] | ||
| Acidity (pKa) | 13.57 (25 °C, H2O)[6] | ||
| -.5153−6 cm3/g | |||
Refractive index (nD) |
1.3316 | ||
| Viscosity | 0.21 mPa-s at 20 °C (0.253 mPa-s at 9.5 °C) [7] | ||
| Structure | |||
| trigonal planar (sp²) at C1 tetrahedral (sp³) at C2 | |||
| 2.7 D | |||
| Thermochemistry | |||
Std molar entropy (S |
250 J·mol−1·K−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−166 kJ·mol−1 | ||
| Hazards | |||
| Main hazards | potential occupational carcinogen[8] | ||
| Safety data sheet | See: data page HMDB | ||
| GHS pictograms |    | ||
GHS hazard statements |
H224, H319, H335, H351[9] | ||
| P210, P261, P281, P305+351+338[9] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −39.00 °C; −38.20 °F; 234.15 K | ||
| 175.00 °C; 347.00 °F; 448.15 K[5] | |||
| Explosive limits | 4.0–60% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
1930 mg/kg (rat, oral) | ||
LC50 (median concentration) |
13,000 ppm (rat), 17,000 ppm (hamster), 20,000 ppm (rat)[8] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
200 ppm (360 mg/m3)[5] | ||
IDLH (Immediate danger) |
2000 ppm[5][8] | ||
| Related compounds | |||
Related aldehydes |
Formaldehyde Propionaldehyde | ||
Related compounds |
Ethylene oxide | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen.[12] Acetaldehyde is "one of the most frequently found air toxins with cancer risk greater than one in a million".[13]
History
Acetaldehyde was first observed by the Swedish pharmacist/chemist Carl Wilhelm Scheele (1774);[14] it was then investigated by the French chemists Antoine François, comte de Fourcroy and Louis Nicolas Vauquelin (1800),[15] and the German chemists Johann Wolfgang Döbereiner (1821, 1822, 1832)[16] and Justus von Liebig (1835).[17][18] In 1835, Liebig named it "aldehyde";[19] the name was later altered to "acetaldehyde".[20]
Production
In 2003, global production was about 1 million tonnes. Before 1962, ethanol and acetylene were the major sources of acetaldehyde. Since then, ethylene is the dominant feedstock.[21]
The main method of production is the oxidation of ethylene by the Wacker process, which involves oxidation of ethylene using a homogeneous palladium/copper system:
- 2 CH2=CH2 + O2 → 2 CH3CHO
In the 1970s, the world capacity of the Wacker-Hoechst direct oxidation process exceeded 2 million tonnes annually.
Smaller quantities can be prepared by the partial oxidation of ethanol in an exothermic reaction. This process typically is conducted over a silver catalyst at about 500–650 °C.[21]
- CH3CH2OH + 1⁄2 O2 → CH3CHO + H2O
This method is one of the oldest routes for the industrial preparation of acetaldehyde.
Other methods
Hydration of acetylene
Prior to the Wacker process and the availability of cheap ethylene, acetaldehyde was produced by the hydration of acetylene.[22] This reaction is catalyzed by mercury(II) salts:
- C2H2 + Hg2+ + H2O → CH3CHO + Hg
The mechanism involves the intermediacy of vinyl alcohol, which tautomerizes to acetaldehyde. The reaction is conducted at 90–95 °C, and the acetaldehyde formed is separated from water and mercury and cooled to 25–30 °C. In the wet oxidation process, iron(III) sulfate is used to reoxidize the mercury back to the mercury(II) salt. The resulting iron(II) sulfate is oxidized in a separate reactor with nitric acid.[21]
Dehydrogenation of ethanol
Traditionally, acetaldehyde was produced by the partial dehydrogenation of ethanol:
- CH3CH2OH → CH3CHO + H2
In this endothermic process, ethanol vapor is passed at 260–290 °C over a copper-based catalyst. The process was once attractive because of the value of the hydrogen coproduct,[21] but in modern times is not economically viable.
Hydroformylation of methanol
The hydroformylation of methanol with catalysts like cobalt, nickel, or iron salts also produces acetaldehyde, although this process is of no industrial importance. Similarly noncompetitive, acetaldehyde arises from synthesis gas with modest selectivity.[21]
Reactions
Tautomerization of acetaldehyde to vinyl alcohol

Like many other carbonyl compounds, acetaldehyde tautomerizes to give an enol (vinyl alcohol; IUPAC name: ethenol):
- CH3CH=O ⇌ CH2=CHOH ∆H298,g = +42.7 kJ/mol
The equilibrium constant is 6×10−7 at room temperature, thus that the relative amount of the enol form in a sample of acetaldehyde is very small.[23] At room temperature, acetaldehyde (CH3CH=O) is more stable than vinyl alcohol (CH2=CHOH) by 42.7 kJ/mol:[24] Overall the keto-enol tautomerization occurs slowly but is catalyzed by acids.
Photo-induced keto-enol tautomerization is viable under atmospheric or stratospheric conditions. This photo-tautomerization is relevant to the earth's atmosphere, because vinyl alcohol is thought to be a precursor to carboxylic acids in the atmosphere.[25][26]
Condensation reactions
Acetaldehyde is a common electrophile in organic synthesis.[27] In condensation reactions, acetaldehyde is prochiral. It is used primarily as a source of the "CH3C+H(OH)" synthon in aldol and related condensation reactions.[28] Grignard reagents and organolithium compounds react with MeCHO to give hydroxyethyl derivatives.[29] In one of the more spectacular condensation reactions, three equivalents of formaldehyde add to MeCHO to give pentaerythritol, C(CH2OH)4.[30]
In a Strecker reaction, acetaldehyde condenses with cyanide and ammonia to give, after hydrolysis, the amino acid alanine.[31] Acetaldehyde can condense with amines to yield imines; for example, with cyclohexylamine to give N-ethylidenecyclohexylamine. These imines can be used to direct subsequent reactions like an aldol condensation.[32]
It is also a building block in the synthesis of heterocyclic compounds. In one example, it converts, upon treatment with ammonia, to 5-ethyl-2-methylpyridine ("aldehyde-collidine").[33]
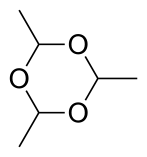
Acetal derivatives
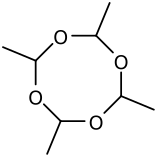
Three molecules of acetaldehyde condense to form "paraldehyde", a cyclic trimer containing C-O single bonds. Similarly condensation of four molecules of acetaldehyde give the cyclic molecule metaldehyde. Paraldehyde can be produced in good yields, using a sulfuric acid catalyst. Metaldehyde is only obtained in a few percent yield and with cooling, often using HBr rather than H2SO4 as the catalyst. At -40 °C in the presence of acid catalysts, polyacetaldehyde is produced.[21]
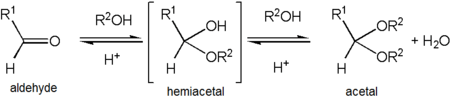
Acetaldehyde forms a stable acetal upon reaction with ethanol under conditions that favor dehydration. The product, CH3CH(OCH2CH3)2, is formally named 1,1-diethoxyethane but is commonly referred to as "acetal".[34] This can cause confusion as "acetal" is more commonly used to describe compounds with the functional groups RCH(OR')2 or RR'C(OR'')2 rather than referring to this specific compound – in fact, 1,1-diethoxyethane is also described as the diethyl acetal of acetaldehyde.
Precursor to vinylphosphonic acid
Acetaldehyde is a precursor to vinylphosphonic acid, which is used to make adhesives and ion conductive membranes. The synthesis sequence begins with a reaction with phosphorus trichloride:[35]
- PCl3 + CH3CHO → CH3CH(O−)PCl3+
- CH3CH(O−)PCl3+ + 2 CH3CO2H → CH3CH(Cl)PO(OH)2 + 2 CH3COCl
- CH3CH(Cl)PO(OH)2 → CH2=CHPO(OH)2 + HCl
Biochemistry
In the liver, the enzyme alcohol dehydrogenase oxidizes ethanol into acetaldehyde, which is then further oxidized into harmless acetic acid by acetaldehyde dehydrogenase. These two oxidation reactions are coupled with the reduction of NAD+ to NADH.[36] In the brain, the enzyme catalase is primarily responsible for oxidizing ethanol to acetaldehyde, and alcohol dehydrogenase plays a minor role.[36] The last steps of alcoholic fermentation in bacteria, plants, and yeast involve the conversion of pyruvate into acetaldehyde and carbon dioxide by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehydrogenase, now operating in the opposite direction.
Uses
Traditionally, acetaldehyde was mainly used as a precursor to acetic acid. This application has declined because acetic acid is produced more efficiently from methanol by the Monsanto and Cativa processes. Acetaldehyde is an important precursor to pyridine derivatives, pentaerythritol, and crotonaldehyde. Urea and acetaldehyde combine to give a useful resin. Acetic anhydride reacts with acetaldehyde to give ethylidene diacetate, a precursor to vinyl acetate, which is used to produce polyvinyl acetate.[21]
The global market for acetaldehyde is declining. Demand has been impacted by changes in the production of plasticizer alcohols, which has shifted because n-butyraldehyde is less often produced from acetaldehyde, instead being generated by hydroformylation of propylene. Likewise, acetic acid, once produced from acetaldehyde, is made predominantly by the lower-cost methanol carbonylation process.[37] The impact on demand has led to increase in prices and thus slowdown in the market.
Consumption of acetaldehyde (103 t) in 2003[21]
(* Included in others -glyoxal/glyoxalic acid, crotonaldehyde, lactic acid, n-butanol, 2-ethylhexanol)
| Product | USA | Mexico | W. Europe | Japan | Total |
|---|---|---|---|---|---|
| Acetic Acid/Acetic anhydride | - | 11 | 89 | 47 | 147 |
| Acetate esters | 35 | 8 | 54 | 224 | 321 |
| Pentaerythritol | 26 | – | 43 | 11 | 80 |
| Pyridine and pyridine bases | 73 | – | 10 | * | 83 |
| Peracetic acid | 23 | – | – | * | 23 |
| 1,3-Butylene glycol | 14 | – | – | * | 14 |
| Others | 5 | 3 | 10 | 80 | 98 |
| Total | 176 | 22 | 206 | 362 | 766 |
China is the largest consumer of acetaldehyde in the world, accounting for almost half of global consumption in 2012. Major use has been the production of acetic acid. Other uses such as pyridines and pentaerythritol are expected to grow faster than acetic acid, but the volumes are not large enough to offset the decline in acetic acid. As a consequence, overall acetaldehyde consumption in China may grow slightly at 1.6% per year through 2018. Western Europe is the second-largest consumer of acetaldehyde worldwide, accounting for 20% of world consumption in 2012. As with China, the Western European acetaldehyde market is expected to increase only very slightly at 1% per year during 2012–2018. However, Japan could emerge as a potential consumer for acetaldehyde in next five years due to newfound use in commercial production of butadiene. The supply of butadiene has been volatile in Japan and the rest of Asia. This should provide the much needed boost to the flat market, as of 2013.[38]
Safety
Exposure limits
The threshold limit value is 25ppm (STEL/ceiling value) and the MAK (Maximum Workplace Concentration) is 50 ppm. At 50 ppm acetaldehyde, no irritation or local tissue damage in the nasal mucosa is observed. When taken up by the organism, acetaldehyde is metabolized rapidly in the liver to acetic acid. Only a small proportion is exhaled unchanged. After intravenous injection, the half-life in the blood is approximately 90 seconds.[21]
Dangers
Toxicity
No serious cases of acute intoxication have been recorded.[21] Acetaldehyde naturally breaks down in the human body[11] but has been shown to excrete in urine of rats.[39]
Irritation
Acetaldehyde is an irritant of the skin, eyes, mucous membranes, throat, and respiratory tract. This occurs at concentrations as low as 1000 ppm. Symptoms of exposure to this compound include nausea, vomiting, and headache. These symptoms may not happen immediately. The perception threshold for acetaldehyde in air is in the range between 0.07 and 0.25 ppm.[21] At such concentrations, the fruity odor of acetaldehyde is apparent. Conjunctival irritations have been observed after a 15-minute exposure to concentrations of 25 and 50 ppm, but transient conjunctivitis and irritation of the respiratory tract have been reported after exposure to 200 ppm acetaldehyde for 15 minutes.
Carcinogenicity
Acetaldehyde is carcinogenic in humans.[40][41] In 1988 the International Agency for Research on Cancer stated, "There is sufficient evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals."[42] In October 2009 the International Agency for Research on Cancer updated the classification of acetaldehyde stating that acetaldehyde included in and generated endogenously from alcoholic beverages is a Group I human carcinogen.[43] In addition, acetaldehyde is damaging to DNA[44] and causes abnormal muscle development as it binds to proteins.[45]
Aggravating factors
Alzheimer's disease
People with a genetic deficiency for the enzyme responsible for the conversion of acetaldehyde into acetic acid may have a greater risk of Alzheimer's disease. "These results indicate that the ALDH2 deficiency is a risk factor for LOAD [late-onset Alzheimer's disease] ..."[46]
Genetic conditions
A study of 818 heavy drinkers found that those exposed to more acetaldehyde than normal through a defect in the gene for acetaldehyde dehydrogenase are at greater risk of developing cancers of the upper gastrointestinal tract and liver.[47]
Disulfiram
The drug disulfiram (Antabuse) prevents the oxidation of acetaldehyde to acetic acid. Antabuse is sometimes used as a deterrent for alcoholics wishing to stay sober.
Sources of exposure
Indoor air
Acetaldehyde is a potential contaminant in workplace, indoors, and ambient environments. Moreover, the majority of humans spend more than 90% of their time in indoor environments, increasing any exposure and the risk to human health.[48]
In a study in France, the mean indoor concentration of acetaldehydes measured in 16 homes was approximately seven times higher than the outside acetaldehyde concentration. The living room had a mean of 18.1±17.5 μg m−3 and the bedroom was 18.2±16.9 μg m−3, whereas the outdoor air had a mean concentration of 2.3±2.6 μg m−3.
It has been concluded that volatile organic compounds (VOC) such as benzene, formaldehyde, acetaldehyde, toluene, and xylenes have to be considered priority pollutants with respect to their health effects. It has been pointed that in renovated or completely new buildings, the VOCs concentration levels are often several orders of magnitude higher. The main sources of acetaldehydes in homes include building materials, laminate, linoleum, wooden varnished, and cork/pine flooring. It is also found in plastic water-based and matt emulsion paints, in wood ceilings, and wooden, particle-board, plywood, pine wood, and chipboard furniture.[49]
Outdoor air
The use of acetaldehyde is widespread in different industries, and it may be released into waste water or the air during production, use, transportation and storage. Sources of acetaldehyde include fuel combustion emissions from stationary internal combustion engines and power plants that burn fossil fuels, wood, or trash, oil and gas extraction, refineries, cement kilns, lumber and wood mills and paper mills. Acetaldehyde is also present in automobile and diesel exhaust.[50] As a result, acetaldehyde is "one of the most frequently found air toxics with cancer risk greater than one in a million".[13]
Tobacco smoke
Natural tobacco polysaccharides, including cellulose, have been shown to be the primary precursors making acetaldehyde a significant constituent of tobacco smoke.[51][52] It has been demonstrated to have a synergistic effect with nicotine in rodent studies of addiction.[53][54] Acetaldehyde is also the most abundant carcinogen in tobacco smoke; it is dissolved into the saliva while smoking.
Cannabis smoke
Acetaldehyde has been found in cannabis smoke. This finding emerged through the use of new chemical techniques that demonstrated the acetaldehyde present was causing DNA damage in laboratory settings.[55]
Alcohol consumption
Many microbes produce acetaldehyde from ethanol, but they have a lower capacity to eliminate the acetaldehyde, which can lead to the accumulation of acetaldehyde in saliva, stomach acid, and intestinal contents. Fermented food and many alcoholic beverages can also contain significant amounts of acetaldehyde. Acetaldehyde, derived from mucosal or microbial oxidation of ethanol, tobacco smoke, and diet, appears to act as a cumulative carcinogen in the upper digestive tract of humans.[56] According to European Commission's Scientific Committee on Consumer Safety's (SCCS) "Opinion on Acetaldehyde" (2012) the cosmetic products special risk limit is 5 mg/l and acetaldehyde should not be used in mouth-washing products.[57]
Plastics
Acetaldehyde is also created by thermal degradation or ultraviolet photo-degradation of some thermoplastic polymers during or after manufacture. One common example occurs when a bottle of water is left in a hot car for a few hours on a hot, sunny day, and one notices its strange sweet taste in the water from the breakdown of the polyethylene terephthalate (PETE) container.[58] The water industry generally recognizes 20–40 ppb as the taste/odor threshold for acetaldehyde. The level at which an average consumer could detect acetaldehyde is still considerably lower than any toxicity.[59]
Candida Overgrowth
Candida albicans in patients with potentially carcinogenic oral diseases has been shown to produce acetaldehyde in quantities sufficient to cause problems.[60]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 908. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- SciFinderScholar (accessed 4 Nov 2009). Acetaldehyde (75-07-0) Substance Detail.
- Molecular Pathology and Diagnostics of Cancer p.190
- Stoffdaten Acetaldehyd bei Celanese Chemicals. Archived 17 May 2008 at the Wayback Machine as of December 1999.
- NIOSH Pocket Guide to Chemical Hazards. "#0001". National Institute for Occupational Safety and Health (NIOSH).
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–88. ISBN 9781498754293.
- "Acetaldehyde".
- "Acetaldehyde". NIOSH. 4 December 2014. Retrieved 12 February 2015.
- Sigma-Aldrich Co., Acetaldehyde. Retrieved on 2013-07-20.
- Uebelacker, Michael; Lachenmeier, Dirk (13 June 2011). "Quantitative Determination of Acetaldehyde in Foods Using Automated Digestion with Simulated Gastric Fluid Followed by Headspace Gas Chromatography". Journal of Automated Methods and Management in Chemistry. 2011: 907317. doi:10.1155/2011/907317. PMC 3124883. PMID 21747735.
- "Chemicals in the Environment: Acetaldehyde (CAS NO. 75-07-0)". epa.gov. Office of Pollution Prevention and Toxics, United States Environmental Protection Agency. August 1994. Archived from the original on 17 August 2002. Retrieved 22 January 2011.
- List of IARC Group 1 carcinogens
- Zhou, Ying; Li, Chaoyang; Huijbregts, Mark A. J.; Mumtaz, M. Moiz (7 October 2015). "Carcinogenic Air Toxics Exposure and Their Cancer-Related Health Impacts in the United States". PLOS One. 10 (10): e0140013. Bibcode:2015PLoSO..1040013Z. doi:10.1371/journal.pone.0140013. PMC 4596837. PMID 26444872.
- Scheele, C. W. (1774) "Om Brunsten eller Magnesia nigra och dess egenskaper" (On brown-stone or black magnesia [i.e., manganese ore] and its properties), Kungliga Svenska vetenskapsakademiens handlingar (Proceedings of the Royal Swedish Academy of Sciences), 35 : 89–116; 177–194. On pages 109–110, Scheele mentions that refluxing ("digesting") ethanol (Alkohol vini) with manganese dioxide (Brunsten) and either hydrochloric acid (Spirtus salis) or sulfuric acid (Spiritus Vitrioli) produces a smell like "Aether nitri" (ethanol treated with nitric acid). Later investigators realized that Scheele had produced acetaldehyde.
- Note:
- Dabit, a pharmacist in Nantes, France, performed a series of experiments and concluded that acetaldehyde was formed when hydrogen in ethanol combined with oxygen in sulfuric acid to form water: Dabit (1800) "Extrait du mémoire du cit. Dabit sur l'éther" (Extract of the memoir by citizen Dabit on ether), Annales de Chimie, 34 : 289–305.
- Fourcroy and Vauquelin stated that sulfuric acid was not consumed in the production of acetaldehyde: Fourcroy and Vauquelin (1800), "Sur l'éther préparé à la manière du cit. Dabit" (On the ether prepared in the way of citizen Dabit), Annales de Chimie, 34 : 318-332.
- See:
- (Döbereiner) (1821) "Neue Aether" (A new ether), Journal für Chemie und Physik, 32 : 269–270. Döbereiner named the new "ether" "Sauerstoffäther" (oxygen-ether).
- (Döbereiner) (1822) "Döbereiner's Apparat zur Darstellung des Sauerstoffaethers" (Döbereiner's apparatus for the preparation of oxygen-ether), Journal für Chemie und Physik, 34 : 124–125.
- Döbereiner, J. W. (1832) "Bildung des Sauerstoff-Aethers durch atmosphärische Oxidation des Alkohols" (Formation of oxy-ether by atmospheric oxidation of alcohol), Journal für Chemie und Physik, 64 : 466–468. In this paper, Döbereiner made acetaldehyde by exposing ethanol vapor to air in the presence of platinum black.
- Liebig, Justus (1835) "Ueber die Producte der Oxydation des Alkohols" (On the products of oxidation of alcohol [i.e., ethanol]), Annalen der Chemie, 14 : 133–167.
- Brock, William H. (1997) Justus von Liebig: The Chemical Gatekeeper. Cambridge, England: Cambridge University Press, pp. 83–84.
- Liebig, J. (1835) "Sur les produits de l'oxidation de l'alcool" (On the products of the oxidation of alcohol), Annales de Chimie et de Physique, 59 : 289–327. From p. 290: "Je le décrirai dans ce mémoire sous le nom d'aldehyde; ce nom est formé de alcool dehydrogenatus." (I will describe it in this memoir by the name of aldehyde; this name is formed from alcohol dehydrogenatus.)
- The name change occurred at least as early as 1868. See, for example: Eugen F. von Gorup-Besanez, ed., Lehrbuch der organischen Chemie für den Unterricht auf Universitäten ... [Textbook of Organic Chemistry for Instruction at Universities ...], 3rd ed. (Braunschweig, Germany: Friedrich Vieweg und Sohn, 1868), vol. 2, p. 88.
- Eckert, Marc et al. (2007) "Acetaldehyde" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_031.pub2
- Dmitry A. Ponomarev; Sergey M. Shevchenko (2007). "Hydration of Acetylene: A 125th Anniversary" (PDF). J. Chem. Educ. 84 (10): 1725. Bibcode:2007JChEd..84.1725P. doi:10.1021/ed084p1725.
- Keeffe, J. R.; Kresge, A. J.; Schepp, N. P. (1990). "Keto-enol equilibrium constants of simple monofunctional aldehydes and ketones in aqueous solution". Journal of the American Chemical Society. 112 (12): 4862–4868. doi:10.1021/ja00168a035.
- Johnson, R.D. III "CCCBDB NIST Standard Reference Database". nist.gov
- Heazlewood, B. R.; MacCarone, A. T.; Andrews, D. U.; Osborn, D. L.; Harding, L. B.; Klippenstein, S. J.; Jordan, M. J. T.; Kable, S. H. (2011). "Near-threshold H/D exchange in CD3CHO photodissociation". Nature Chemistry. 3 (6): 443–8. Bibcode:2011NatCh...3..443H. doi:10.1038/nchem.1052. PMID 21602858.
- Andrews, D. U.; Heazlewood, B. R.; MacCarone, A. T.; Conroy, T.; Payne, R. J.; Jordan, M. J. T.; Kable, S. H. (2012). "Photo-Tautomerization of Acetaldehyde to Vinyl Alcohol: A Potential Route to Tropospheric Acids". Science. 337 (6099): 1203–6. Bibcode:2012Sci...337.1203A. doi:10.1126/science.1220712. PMID 22903524.
- Sowin, T. J.; Melcher, L. M. (2004) "Acetaldehyde" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette), J. Wiley & Sons, New York. doi:10.1002/047084289X
- Behrens, C.; Paquette, L. A. (1998). "N-Benzyl-2,3-Azetidinedione (2,3-Azetidinedione, 1-(phenylmethyl)-)". Organic Syntheses. 75: 106. doi:10.15227/orgsyn.075.0106.; Collective Volume, 10, p. 41.
- Walter, L. A. (1943). "1-(α-Pyridyl)-2-Propanol (2-(β-Hydroxypropyl)pyridine)". Organic Syntheses. 23: 83. doi:10.15227/orgsyn.023.0083.; Collective Volume, 3, p. 757
- Schurink, H. B. J. (1925). "Pentaerythritol". Organic Syntheses. 4: 53. doi:10.15227/orgsyn.004.0053.; Collective Volume, 1, p. 425
- Kendall, E. C.; McKenzie, B. F. (1929). "dl-Alanine". Organic Syntheses. 9: 4. doi:10.15227/orgsyn.009.0004.; Collective Volume, 1, p. 21
- Wittig, G.; Hesse, A. (1970). "Directed Aldol Condensations: β-Phenylcinnamaldehyde (2-Propenal, 3,3-diphenyl-)". Organic Syntheses. 50: 66. doi:10.15227/orgsyn.050.0066.; Collective Volume, 6, p. 901
- Frank, R. L.; Pilgrim, F. J.; Riener, E. F. (1950). "5-Ethyl-2-Methylpyridine (2-Picoline, 5-ethyl-)". Organic Syntheses. 30: 41. doi:10.15227/orgsyn.030.0041.; Collective Volume, 4, p. 451
- Adkins, H.; Nissen, B. H. (1923). "Acetal". Organic Syntheses. 3: 1. doi:10.15227/orgsyn.003.0001.; Collective Volume, 1, p. 1
- Lavinia, M.; Gheorghe, I. (2010). "Poly(vinylphosphonic acid) and its derivatives". Progress in Polymer Science. 35 (8): 1078–1092. doi:10.1016/j.progpolymsci.2010.04.001.
- Hipolito, L.; Sanchez, M. J.; Polache, A.; Granero, L. (2007). "Brain metabolism of ethanol and alcoholism: An update". Curr. Drug Metab. 8 (7): 716–727. doi:10.2174/138920007782109797. PMID 17979660.
- "Acetaldehyde". ihs.com.
- Research and Markets ltd. "Acetaldehyde – Global Business Strategic Report".
- Tsukamoto, S; Muto, T; Nagoya, T; Shimamura, M; Saito, M; Tainaka, H (1989). "Determinations of ethanol, acetaldehyde and acetate in blood and urine during alcohol oxidation in man". Alcohol and Alcoholism. 24 (2): 101–8. doi:10.1093/oxfordjournals.alcalc.a044872. PMID 2719768.
- Chemical Summary For Acetaldehyde, US Environmental Protection Agency
- Scientific Committee on Cosmetic Products and Non-Food Products (25 May 2004). "Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers Concerning Acetaldehyde" (PDF). p. 11. Retrieved 28 September 2011.
- International Agency for Rescarch on Cancer, World Health Organization. (1988). Alcohol drinking. Lyon: World Health Organization, International Agency for Research on Cancer. ISBN 978-92-832-1244-7. p3
- International Agency for Research on Cancer Monograph Working Group, Special Report: Policy A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. The Lancet 2009 10, 1033–1034.
- Lambert, B; He, S. M. (1988). "DNA and chromosome damage induced by acetaldehyde in human lymphocytes in vitro". Annals of the New York Academy of Sciences. 534 (1): 369–76. Bibcode:1988NYASA.534..369L. doi:10.1111/j.1749-6632.1988.tb30124.x. PMID 3389666.
- Aberle, N. S.; Burd, L; Zhao, B. H.; Ren, J (2004). "Acetaldehyde-Induced Cardiac Contractile Dysfunction May Be Alleviated by Vitamin B1 but Not by Vitamins B6 or B12". Alcohol and Alcoholism. 39 (5): 450–4. doi:10.1093/alcalc/agh085. PMID 15304379.
- Ohta, S; Ohsawa I; Kamino K; Ando F; Shimokata H. (April 2004). "Mitochondrial ALDH2 Deficiency as an Oxidative Stress". Annals of the New York Academy of Sciences. 1011 (1): 36–44. Bibcode:2004NYASA1011...36O. doi:10.1196/annals.1293.004. PMID 15126281.
- Homann, N.; Stickel, F.; König, I. R.; Jacobs, A.; Junghanns, K.; Benesova, M.; Schuppan, D.; Himsel, S.; Zuber-Jerger, I.; Hellerbrand, C.; Ludwig, D.; Caselmann, W. H.; Seitz, H. K. (2006). "Alcohol dehydrogenase 1C*1 allele is a genetic marker for alcohol-associated cancer in heavy drinkers". International Journal of Cancer. 118 (8): 1998–2002. doi:10.1002/ijc.21583. PMID 16287084.
- Spengler, John D.; McCarthy, John F.; Samet, Jonathan M. (2000). Indoor Air Quality Handbook. New York, NY, USA: McGraw-Hill Professional Publishing. p. 761. ISBN 978-0074455494.
- Dafni A. Missia; E. Demetriou; N. Michael; E.I. Tolis; J.G. Bartzis (2010). "Indoor exposure from building materials: A field study". Atmospheric Environment. 44 (35): 4388–4395. Bibcode:2010AtmEn..44.4388M. doi:10.1016/j.atmosenv.2010.07.049.
- Clements, A. L.; Jia, Y.; Denbleyker, A.; McDonald-Buller, E.; Fraser, M. P.; Allen, D. T.; Collins, D. R.; Michel, E.; Pudota, J.; Sullivan, D.; Zhu, Y. (2009). "Air pollutant concentrations near three Texas roadways, part II: Chemical characterization and transformation of pollutants". Atmospheric Environment. 43 (30): 4523–4534. Bibcode:2009AtmEn..43.4523C. doi:10.1016/j.atmosenv.2009.06.044.
- Talhout, R; Opperhuizen, A; van Amsterdam, JG (October 2007). "Role of acetaldehyde in tobacco smoke addiction". Eur Neuropsychopharmacol. 17 (10): 627–36. doi:10.1016/j.euroneuro.2007.02.013. PMID 17382522.
- Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
- "NIDA – Publications – NIDA Notes – Vol. 20, No. 3" Archived 25 August 2009 at the Wayback Machine. drugabuse.gov.
- Nicotine's addictive hold increases when combined with other tobacco smoke chemicals, UCI study finds. University of California. 2004-10-28
- Singh, R (2009). "Evaluation of the DNA Damaging Potential of Cannabis Cigarette Smoke by the Determination of Acetaldehyde Derived N2-Ethyl-2′-deoxyguanosine Adducts". Chem. Res. Toxicol. 22 (6): 1181–1188. doi:10.1021/tx900106y. PMID 19449825.
- Salaspuro, M. (2009). "Acetaldehyde as a common denominator and cumulative carcinogen in digestive tract cancers". Scandinavian Journal of Gastroenterology. 44 (8): 912–925. doi:10.1080/00365520902912563. PMID 19396661.
- Scientific Committee on Consumer Safety SCCS OPINION ON Acetaldehyde. European Commission. 18 September 2012
- Dornath, Paul John (2010). "Analysis of Chemical Leaching from Common Consumer Plastic Bottles Under High Stress Conditions" (PDF). p. 32. Archived from the original (PDF) on 26 February 2015. Retrieved 26 February 2015.
- "Do Acetaldehyde and Formaldehyde from Pet Bottles Result in Unacceptable Flavor or Aroma in Bottled Water?" (PDF). PET Resin Association. Retrieved 26 February 2015.
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. (March 2013). "Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders". Journal of Oral Pathology and Medicine. 42 (3): 243–249. doi:10.1111/j.1600-0714.2012.01203.x. PMID 22909057.
External links
| Wikimedia Commons has media related to Acetaldehyde. |
- International Chemical Safety Card 0009
- NIOSH Pocket Guide to Chemical Hazards
- Methods for sampling and analysis
- IARC Monograph: "Acetaldehyde"
- Hal Kibbey, Genetic Influences on Alcohol Drinking and Alcoholism, Indiana University Research and Creative Activity, Vol. 17 no. 3.
- United States Food and Drug Administration (FDA) information for acetaldehyde
- Acetaldehyde production process flow sheet by ethylene oxidation method