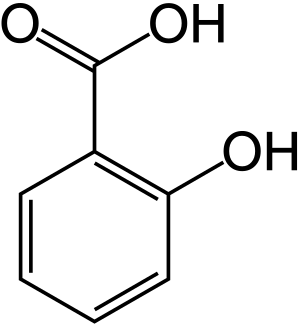
Salicylic acid
Salicylic acid (from Latin salix, willow tree) is a lipophilic monohydroxybenzoic acid, a type of phenolic acid, and a beta hydroxy acid (BHA). It has the formula C7H6O3. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin.
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxybenzoic acid[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.648 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6O3 | |||
| Molar mass | 138.122 g·mol−1 | ||
| Appearance | Colorless to white crystals | ||
| Odor | Odorless | ||
| Density | 1.443 g/cm3 (20 °C)[2] | ||
| Melting point | 158.6 °C (317.5 °F; 431.8 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) decomposes[3] 211 °C (412 °F; 484 K) at 20 mmHg[2] | ||
| Sublimes at 76 °C[4] | |||
| Solubility | Soluble in ether, CCl4, benzene, propanol, acetone, ethanol, oil of turpentine, toluene | ||
| Solubility in benzene | |||
| Solubility in chloroform | |||
| Solubility in methanol |
| ||
| Solubility in olive oil | 2.43 g/100 g (23 °C)[3] | ||
| Solubility in acetone | 39.6 g/100 g (23 °C)[3] | ||
| log P | 2.26 | ||
| Vapor pressure | 10.93 mPa[4] | ||
| Acidity (pKa) | |||
| UV-vis (λmax) | 210 nm, 234 nm, 303 nm (4 mg % in ethanol)[4] | ||
| −72.23·10−6 cm3/mol | |||
Refractive index (nD) |
1.565 (20 °C)[2] | ||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
−589.9 kJ/mol | ||
Std enthalpy of combustion (ΔcH⦵298) |
3.025 MJ/mol[7] | ||
| Pharmacology | |||
| A01AD05 (WHO) B01AC06 (WHO) D01AE12 (WHO) N02BA01 (WHO) S01BC08 (WHO) | |||
| Hazards | |||
| Safety data sheet | MSDS | ||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H302, H318[8] | ||
| P280, P305+351+338[8] | |||
| Eye hazard | Severe irritation | ||
| Skin hazard | Mild irritation | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 157 °C (315 °F; 430 K) closed cup[4] | ||
| 540 °C (1,004 °F; 813 K)[4] | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
480 mg/kg (mice, oral) | ||
| Related compounds | |||
Related compounds |
Methyl salicylate, Benzoic acid, Phenol, Aspirin, 4-Hydroxybenzoic acid, Magnesium salicylate, Choline salicylate, Bismuth subsalicylate, Sulfosalicylic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
In addition to serving as an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prodrug to salicylic acid, it is probably best known for its use as a key ingredient in topical anti-acne products. The salts and esters of salicylic acid are known as salicylates.
It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[9]
Uses
Medicine
Salicylic acid as a medication is used most commonly to help remove the outer layer of the skin.[10] As such, it is used to treat warts, psoriasis, acne, ringworm, dandruff, and ichthyosis.[10][11]
Similar to other hydroxy acids, salicylic acid is a key ingredient in many skincare products for the treatment of seborrhoeic dermatitis, acne, psoriasis, calluses, corns, keratosis pilaris, acanthosis nigricans, ichthyosis and warts.[12]
Uses in manufacturing
Salicylic acid is used in the production of other pharmaceuticals, including 4-aminosalicylic acid, sandulpiride, and landetimide (via Salethamide).
Salicylic acid was one of the original starting materials for making acetylsalicylic acid (aspirin) in 1897.[13]
Bismuth subsalicylate, a salt of bismuth and salicylic acid, is the active ingredient in stomach relief aids such as Pepto-Bismol, is the main ingredient of Kaopectate and "displays anti-inflammatory action (due to salicylic acid) and also acts as an antacid and mild antibiotic".[14]
Other derivatives include methyl salicylate used as a liniment to soothe joint and muscle pain and choline salicylate used topically to relieve the pain of mouth ulcers.
Other uses
Salicylic acid is used as a food preservative, a bactericidal and an antiseptic.[15]
Sodium salicylate is a useful phosphor in the vacuum ultraviolet spectral range, with nearly flat quantum efficiency for wavelengths between 10 and 100 nm.[16] It fluoresces in the blue at 420 nm. It is easily prepared on a clean surface by spraying a saturated solution of the salt in methanol followed by evaporation.
Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.
Mechanism of action
Salicylic acid modulates COX2 gene expression to decrease the formation of pro-inflammatory prostaglandins. Salicylate may competitively inhibit prostaglandin formation. Salicylate's antirheumatic (nonsteroidal anti-inflammatory) actions are a result of its analgesic and anti-inflammatory mechanisms.
Salicylic acid works by causing the cells of the epidermis to slough off more readily, preventing pores from clogging up, and allowing room for new cell growth. Salicylic acid inhibits the oxidation of uridine-5-diphosphoglucose (UDPG) competitively with nicotinamide adenosine dinucleotide (NAD) and noncompetitively with UDPG. It also competitively inhibits the transferring of glucuronyl group of uridine-5-phosphoglucuronic acid (UDPGA) to the phenolic acceptor.
The wound-healing retardation action of salicylates is probably due mainly to its inhibitory action on mucopolysaccharide synthesis.[17]
Safety
17% to 27% salicylic acid used in the form of a paint, and 20% to 50% in plaster form, which are sold for wart and corn removal should not be applied to the face and should not be used for acne treatment.[18] Even for wart removal, such a solution should be applied once or twice a day – more frequent use may lead to an increase in side-effects without an increase in efficacy.[19]
If high concentrations of salicylic ointment are applied to a large percentage of body surface, high levels of salicylic acid can enter the blood, requiring hemodialysis to avoid further complications.[20]
Chemistry and production

Salicylic acid has the formula C6H4(OH)COOH, where the OH group is ortho to the carboxyl group. It is also known as 2-hydroxybenzoic acid. It is poorly soluble in water (2 g/L at 20 °C).[21]
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana it can be synthesized via a phenylalanine-independent pathway.
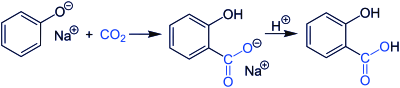
Sodium salicylate is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (115°C) – a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid:
It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid)[22] or methyl salicylate (oil of wintergreen) with a strong acid or base.
Salicylic acid degrades to phenol and carbon dioxide at 200 - 230°C:[23]
- C6H4OH(CO2H) → C6H5OH + CO2
History
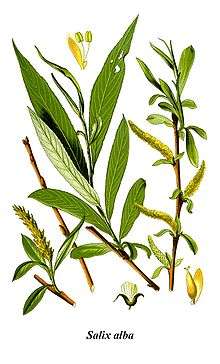
Hippocrates, Galen, Pliny the Elder and others knew that willow bark could ease pain and reduce fevers.[24] It was used in Europe and China to treat these conditions.[25] This remedy is mentioned in texts from ancient Egypt, Sumer and Assyria.[26] The Cherokee and other Native Americans use an infusion of the bark for fever and other medicinal purposes.[27]
In 2014, archaeologists identified traces of salicylic acid on 7th century pottery fragments found in east central Colorado.[28] The Reverend Edward Stone, a vicar from Chipping Norton, Oxfordshire, England, noted in 1763 that the bark of the willow was effective in reducing a fever.[29]
The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1828.[30] A larger amount of the substance was isolated in 1829 by Henri Leroux, a French pharmacist.[31] Raffaele Piria, an Italian chemist, was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.[32][33]
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839.[34] While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea and even death when consumed in high doses.
Dietary sources
Salicylic acid occurs in plants as free salicylic acid and its carboxylated esters and phenolic glycosides. Several studies suggest that humans metabolize salicylic acid in measurable quantities from these plants.[35] High-salicylate beverages and foods include beer, coffee, tea, numerous fruits and vegetables, sweet potato, nuts, and olive oil, among others.[36] Meat, poultry, fish, eggs, dairy products, sugar, and breads and cereals have low salicylate content.[36][37]
Some people with sensitivity to dietary salicylates may have symptoms of allergic reaction, such as bronchial asthma, rhinitis, gastrointestinal disorders, or diarrhea, and so may need to adopt a low-salicylate diet.[36]
Plant hormone
Salicylic acid is a phenolic phytohormone and is found in plants with roles in plant growth and development, photosynthesis, transpiration, ion uptake and transport.[38] Salicylic acid is involved in endogenous signaling, mediating in plant defense against pathogens.[39] It plays a role in the resistance to pathogens by inducing the production of pathogenesis-related proteins.[40]
It is involved in the systemic acquired resistance in which a pathogenic attack on one part of the plant induces resistance in other parts. The signal can also move to nearby plants by salicylic acid being converted to the volatile ester methyl salicylate.[41]Methyl salicylate is taken up by the stomata of the nearby plant, and once deep in the leaf, is converted back to salicylic acid to induce the immune response.[42]
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 64. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.306. ISBN 1439855110.
- "Salicylic acid". Archived from the original on 2014-05-24. Retrieved 2014-05-23.
- CID 338 from PubChem
- Atherton Seidell; William F. Linke (1952). Solubilities of Inorganic and Organic Compounds: A Compilation of Solubility Data from the Periodical Literature. Supplement. Van Nostrand.
- Salicyclic acid Archived 2019-03-27 at the Wayback Machine. Drugbank.ca. Retrieved on 2012-06-03.
- "Salicylic acid". Archived from the original on 2017-02-15. Retrieved 2014-08-17.
- Sigma-Aldrich Co., Salicylic acid. Retrieved on 2014-05-23.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "Salicylic acid". Drugs.com. Archived from the original on 18 January 2017. Retrieved 15 January 2017.
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 310. hdl:10665/44053. ISBN 9789241547659.
- Madan RK; Levitt J (April 2014). "A review of toxicity from topical salicylic acid preparations". J Am Acad Dermatol. 70 (4): 788–92. doi:10.1016/j.jaad.2013.12.005. PMID 24472429.
- Schrör, Karsten (2016). Acetylsalicylic Acid (2 ed.). John Wiley & Sons. pp. 9–10. ISBN 9783527685028.
- "Bismuth subsalicylate". PubChem. United States National Institutes of Health. Archived from the original on 1 February 2014. Retrieved 24 January 2014.
- "Definition of Salicylic acid". MedicineNet.com. Archived from the original on 2011-12-09. Retrieved 2010-10-12.
- Samson, James (1976). Techniques of Vacuum Ultraviolet Spectroscopy. Wiley, .
- "Salicylic Acid". www.drugbank.ca. Archived from the original on 2018-10-29. Retrieved 2018-12-21.
- "SALICYLIC ACID - National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Archived from the original on 2018-08-21. Retrieved 2018-08-21.
- salicylic acid 17 % Topical Liquid. Kaiser Permanente Drug Encyclopedia. Accessed 28 Sept 2011.
- Péc, J.; Strmenová, M.; Palencárová, E.; Pullmann, R.; Funiaková, S.; Visnovský, P.; Buchanec, J.; Lazarová, Z. (October 1992). "Salicylate intoxication after use of topical salicylic acid ointment by a patient with psoriasis". Cutis. 50 (4): 307–309. ISSN 0011-4162. PMID 1424799.
- "Salicylic acid". inchem.org. Archived from the original on 2008-12-18. Retrieved 2008-10-13.
- "Hydrolysis of ASA to SA". Archived from the original on August 8, 2007. Retrieved July 31, 2007.
- Kaeding, Warren W. (1 September 1964). "Oxidation of Aromatic Acids. IV. Decarboxylation of Salicylic Acids". The Journal of Organic Chemistry. 29 (9): 2556–2559. doi:10.1021/jo01032a016.
- Norn, S.; Permin, H.; Kruse, P. R.; Kruse, E. (2009). "[From willow bark to acetylsalicylic acid]". Dansk Medicinhistorisk Årbog (in Danish). 37: 79–98. PMID 20509453.
- "Willow bark". University of Maryland Medical Center. University of Maryland. Archived from the original on 24 December 2011. Retrieved 19 December 2011.
- Goldberg, Daniel R. (Summer 2009). "Aspirin: Turn of the Century Miracle Drug". Chemical Heritage Magazine. 27 (2): 26–30. Archived from the original on 20 March 2018. Retrieved 24 March 2018.
- Hemel, Paul B. and Chiltoskey, Mary U. Cherokee Plants and Their Uses – A 400 Year History, Sylva, NC: Herald Publishing Co. (1975); cited in Dan Moerman, A Database of Foods, Drugs, Dyes and Fibers of Native American Peoples, Derived from Plants. Archived 2007-12-06 at the Wayback Machine A search of this database for "salix AND medicine" finds 63 entries.
- "1,300-Year-Old Pottery Found in Colorado Contains Ancient 'Natural Aspirin'". Archived from the original on 2014-08-13. Retrieved 2014-08-13.
- Stone, Edmund (1763). "An Account of the Success of the Bark of the Willow in the Cure of Agues". Philosophical Transactions of the Royal Society of London. 53: 195–200. doi:10.1098/rstl.1763.0033.
- Buchner, A. (1828). "Ueber das Rigatellische Fiebermittel und über eine in der Weidenrinde entdeckte alcaloidische Substanz (On Rigatelli's antipyretic [i.e., anti-fever drug] and on an alkaloid substance discovered in willow bark)". Repertorium für die pharmacie…. Bei J. L. Schrag. pp. 405–.
Noch ist es mir aber nicht geglückt, den bittern Bestandtheil der Weide, den ich Salicin nennen will, ganz frei von allem Färbestoff darzustellen." (I have still not succeeded in preparing the bitter component of willow, which I will name salicin, completely free from colored matter
- See:
- Leroux, H. (1830). "Mémoire relatif à l'analyse de l'écorce de saule et à la découverte d'un principe immédiat propre à remplacer le sulfate de quinine"] (Memoir concerning the analysis of willow bark and the discovery of a substance immediately likely to replace quinine sulfate)". Journal de Chimie Médicale, de Pharmacie et de Toxicologie. 6: 340–342.
- A report on Leroux's presentation to the French Academy of Sciences also appeared in: Mémoires de l'Académie des sciences de l'Institut de France. Institut de France. 1838. pp. 20–.
- Piria (1838) "Sur de neuveaux produits extraits de la salicine" Archived 2017-07-27 at the Wayback Machine (On new products extracted from salicine), Comptes rendus … 6: 620–624. On page 622, Piria mentions "Hydrure de salicyle" (hydrogen salicylate, i.e., salicylic acid).
- Jeffreys, Diarmuid (2005). Aspirin: the remarkable story of a wonder drug. New York: Bloomsbury. pp. 38–40. ISBN 978-1-58234-600-7.
- Löwig, C.; Weidmann, S. (1839). "III. Untersuchungen mit dem destillierten Wasser der Blüthen von Spiraea Ulmaria (III. Investigations of the water distilled from the blossoms of Spiraea ulmaria). Löwig and Weidman called salicylic acid Spiräasaure (spiraea acid)". Annalen der Physik und Chemie; Beiträge zur Organischen Chemie (Contributions to Organic Chemistry) (46): 57–83.
- Malakar, Sreepurna; Gibson, Peter R.; Barrett, Jacqueline S.; Muir, Jane G. (1 April 2017). "Naturally occurring dietary salicylates: A closer look at common Australian foods". Journal of Food Composition and Analysis. 57: 31–39. doi:10.1016/j.jfca.2016.12.008.
- "Low salicylate diet". Drugs.com. 19 February 2019. Archived from the original on 16 December 2019. Retrieved 16 December 2019.
- Swain, AR; Dutton, SP; Truswell, AS (1985). "Salicylates in foods" (PDF). Journal of the American Dietetic Association. 85 (8): 950–60. ISSN 0002-8223. PMID 4019987. Archived (PDF) from the original on 2019-04-05. Retrieved 2019-12-16.
- Vlot, A. C; Dempsey, D. A; Klessig, D. F (2009). "Salicylic Acid, a multifaceted hormone to combat disease". Annual Review of Phytopathology. 47: 177–206. doi:10.1146/annurev.phyto.050908.135202. PMID 19400653.
- Hayat, S. & Ahmad, A. (2007). Salicylic Acid – A Plant Hormone. Springer. ISBN 978-1-4020-5183-8.
- Van Huijsduijnen, R. A. M. H.; Alblas, S. W.; De Rijk, R. H.; Bol, J. F. (1986). "Induction by Salicylic Acid of Pathogenesis-related Proteins or Resistance to Alfalfa Mosaic Virus Infection in Various Plant Species". Journal of General Virology. 67 (10): 2135–2143. doi:10.1099/0022-1317-67-10-2135.
- Taiz, L. and Zeiger, Eduardo (2002) Plant Physiology Archived 2014-03-05 at the Wayback Machine, 3rd Edition, Sinauer Associates, p. 306, ISBN 0878938230.
- Chamowitz, D. (2017). What a plant knows: a field guide to the senses. Brunswick, Vic.: Scribe Publications.
External links
| Wikimedia Commons has media related to Salicylic acid. |
- Salicylic acid MS Spectrum
- Safety MSDS data
- International Chemical Safety Cards | CDC/NIOSH
- "On the syntheses of salicylic acid": English Translation of Hermann Kolbe's seminal 1860 German article "Ueber Synthese der Salicylsäure" in Annalen der Chemie und Pharmacie at MJLPHD