Homosalate
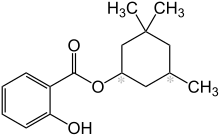
Homosalate is an organic compound used in some sunscreens. It is made by the Fischer–Speier esterification of salicylic acid and 3,3,5-trimethylcyclohexanol, the latter being a hydrogenated derivative of isophorone. Contained in 45% of U.S. sunscreens, it is used as a chemical UV filter.[3] The salicylic acid portion of the molecule absorbs ultraviolet rays with a wavelength from 295 nm to 315 nm, protecting the skin from sun damage. The hydrophobic trimethyl cyclohexane functional group provides greasiness that prevents it from dissolving in water.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3,3,5-Trimethylcyclohexyl 2-hydroxybenzoate | |
| Other names
Homosalate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.874 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H22O3 | |
| Molar mass | 262.349 g·mol−1 |
| Density | 1.05 g/cm3 (20 °C)[2] |
| Melting point | < -20 °C[2] |
| Boiling point | 181–185 °C (358–365 °F; 454–458 K)[2] |
| 0.4 mg/L | |
| Hazards | |
| Flash point | 171 °C (340 °F; 444 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Safety
Similar to other UV filter compounds,[4] more homosalate is absorbed into the stratum corneum of the face (25% of applied dose) versus back of volunteers.[5] Homosalate has been identified as an antiandrogen in vitro,[6] as well as having estrogenic activity toward estrogen receptors α,[7] and general in vitro estrogenic activity.[8] Homosalate has been shown to be an antagonist toward androgen and estrogen receptors in vitro.[9] Some work has shown that organic UV filters in general can present concerns.[10]
No evidence of toxicity or side effects have been documented in vivo.
References
- Homosalate, Merck Index, 11th Edition, 4660.
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Homosalate, ChemIDplus.
- Rougier A, Dupuis D, Lotte C, Roguet R, Wester RC, Maibach HI (1986). "Regional variation in percutaneous absorption in man: measurement by the stripping method". Arch. Dermatol. Res. 278 (6): 465–469. doi:10.1007/bf00455165. PMID 3789805.
- Benson HA, Sarveiya V, Risk S, Roberts MS (2005). "Influence of anatomical site and topical formulation on skin penetration of sunscreens". Ther Clin Risk Manag. 1 (3): 209–218. PMC 1661631. PMID 18360561.
- Ma, R.; Cotton, B.; Lichtensteiger, W.; Schlumpf, M. (2003). "UV Filters with Antagonistic Action at Androgen Receptors in the MDA-kb2 Cell Transcriptional-Activation Assay". Toxicological Sciences. 74 (1): 43–50. doi:10.1093/toxsci/kfg102. PMID 12730620.
- Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, Balaguer P, Casellas C (2005). "Estrogenic activity of cosmetic components in reporter cell lines: parabens, UV screens, and musks". J Toxicol Environ Health A. 68 (4): 239–251. doi:10.1080/15287390590895054. PMID 15799449.
- Schlumpf M, Schmid P, Durrer S, Conscience M, Maerkel K, Henseler M, Gruetter M, Herzog I, Reolon S, Ceccatelli R, Faass O, Stutz E, Jarry H, Wuttke W, Lichtensteiger W (2004). "Endocrine activity and developmental toxicity of cosmetic UV filters--an update". Toxicology. 205 (1–2): 113–122. doi:10.1016/j.tox.2004.06.043. PMID 15458796.
- Schreurs RH, Sonneveld E, Jansen JH, Seinen W, van der Burg B (February 2005). "Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays". Toxicol. Sci. 83 (2): 264–272. doi:10.1093/toxsci/kfi035. PMID 15537743.
- Klimova, et al. (2013). "Current problems in the use of organic UV filters to protect skin from excessive sun exposure" (PDF). Acta Chimica Slovaca. 6 (1): 82–88. doi:10.2478/acs-2013-0014.