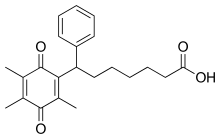
Seratrodast
Seratrodast (development name, AA-2414; marketed originally as Bronica)[2] is a thromboxane A2 (TXA2) receptor (TP receptor) antagonist used primarily in the treatment of asthma.[3][4] It was the first TP receptor antagonist that was developed as an anti-asthmatic drug and received marketing approval in Japan in 1997.[5] As of 2017 seratrodast was marketed as Bronica in Japan, and as Changnuo, Mai Xu Jia, Quan Kang Nuo in China.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Bronica in Japan, Changnuo, Mai Xu Jia, Quan Kang Nuo in China and as Seradair in India. .[1] |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets, granules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >96% |
| Elimination half-life | 22 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.176 |
| Chemical and physical data | |
| Formula | C22H26O4 |
| Molar mass | 354.446 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Unlike thromboxane synthase inhibitors such as ozagrel, seratrodast does not affect thrombus formation, time to occlusion and bleeding time.[6] Seratrodast has no effect on prothrombin time and activated partial thromboplastin time, thus ruling out any action on blood coagulation cascade.[7]
Medical uses
Seratrodast is used to treat asthma.[8][9]
There are no adequate and well-controlled studies of seratrodast in pregnant women. The drug should be used in pregnancy only if the potential benefits justify the risk to the fetus.[9] Seratrodast should not be used during lactation.[9]
The safety and efficacy of seratrodast has not been established in children (<18 years of age).[9]
Contraindications and interactions
Seratrodast should not be used in people with liver disease.[9]
Use with paracetamol or with cephem antibiotics increases the risk of liver damage. Use with aspirin increases the bioavailability of seratrodast.[9]
Adverse effects
The most frequently observed (0.1 to 5%) adverse reactions include elevated transaminases, nausea, loss of appetite, stomach discomfort, abdominal pain, diarrhea, constipation, dry mouth, taste disturbance, drowsiness, headache, dizziness, palpitations and malaise.[9] Less than 0.1% of patients experienced vomiting, thrombocytopenia, epistaxis, bleeding tendency, insomnia, tremor, numbness, hot flushes and edema.[9] All the adverse reactions reported were of mild to moderate severity, and resolved when the drug was discontinued.[9]
Pharmacology
Thromboxane A2 (TXA2) is generated in the lungs of people with asthma, and when it signals through the thromboxane receptor it causes bronchoconstriction, vasoconstriction, mucous secretion, and airway hyper-responsiveness. Seratrodast inhibits the activity of the thromboxane receptor, blocking the effects of TXA2.[10]
Pharmacokinetics
The pharmacokinetics of seratrodast have been studied in Japanese and Caucasian, including Indian, healthy volunteers.[11][12][13][14] The plasma concentrations of seratrodast increase with increasing doses. The absorption of seratrodast is relatively rapid with maximum plasma concentrations of 4.6–6 μg/ml obtained in 3 to 4 hours.[11] Steady state plasma concentrations of seratrodast are reached within 4–5 days.[13] Seratrodast is slowly cleared, mainly by hepatic biotransformation. The drug shows biexponential decay in plasma profiles with a mean elimination half-life of 22 hours.[11][13] Approximately 20% of the administered dose is recovered in the urine, with 60% of the urinary recovery being in the form of conjugates [12]
History
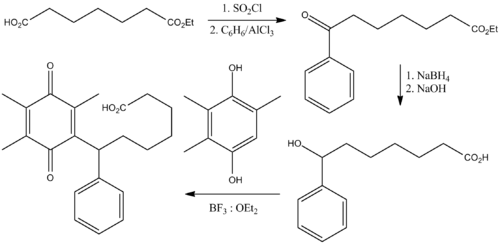
Seratrodast was the first thromboxane receptor antagonist to reach the market as a treatment for asthma; it was approved in Japan in 1997.[8]
Society and culture
As of 2017 seratrodast was marketed as Bronica in Japan, Changnuo, Mai Xu Jia, Quan Kang Nuo in China and as Seretra & Seradair in India.[1]
Research
Seratrodast was studied in perennial allergic rhinitis, chronic bronchitis and chronic pulmonary emphysema but efforts to bring the drug to market in those indications was abandoned around 2000.[2]
References
- "Seratrodast international brands". Drugs.com. Retrieved 8 March 2017.
- "Seratrodast". AdisInsight. Retrieved 8 March 2017.
- Endo S, Akiyama K (November 1996). "[Thromboxane A2 receptor antagonist in asthma therapy]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 54 (11): 3045–8. PMID 8950952.
- Hada S, Hashizume M, Nishii S, Yoshioka F, Yasunaga K (January 1993). "[Study on the inhibitory effect of AA-2414 on platelet aggregation and its clinical effect in asthmatic patients]". Arerugi = [Allergy] (in Japanese). 42 (1): 18–25. PMID 8457165.CS1 maint: extra punctuation (link)
- Dogné JM, de Leval X, Benoit P, Delarge J, Masereel B (2002). "Thromboxane A2 inhibition: therapeutic potential in bronchial asthma". American Journal of Respiratory Medicine. 1 (1): 11–7. doi:10.1007/bf03257158. PMID 14720071.
- Dogné JM, Hanson J, de Leval X, Kolh P, Tchana-Sato V, de Leval L, et al. (May 2004). "Pharmacological characterization of N-tert-butyl-N'-[2-(4'-methylphenylamino)-5-nitrobenzenesulfonyl]urea (BM-573), a novel thromboxane A2 receptor antagonist and thromboxane synthase inhibitor in a rat model of arterial thrombosis and its effects on bleeding time". The Journal of Pharmacology and Experimental Therapeutics. 309 (2): 498–505. doi:10.1124/jpet.103.063610. PMID 14742735.
- Samara EE (1996). "Seratrodast (AA-2414)—A Novel Thromboxane-A2 Receptor Antagonist". Cardiovascular Drug Reviews. 14 (3): 272–85. doi:10.1111/j.1527-3466.1996.tb00231.x.
- Rolin S, Masereel B, Dogné JM (March 2006). "Prostanoids as pharmacological targets in COPD and asthma". European Journal of Pharmacology. 533 (1–3): 89–100. doi:10.1016/j.ejphar.2005.12.058. PMID 16458293.
- "医療用医薬品 : ブロニカ (Japanese label)" (in Japanese). KEGG. October 2016. Retrieved 8 March 2017.
- Dogné JM, de Leval X, Benoit P, Rolin S, Pirotte B, Masereel B (February 2002). "Therapeutic potential of thromboxane inhibitors in asthma". Expert Opinion on Investigational Drugs. 11 (2): 275–81. doi:10.1517/13543784.11.2.275. PMID 11829716.
- An open-labeled, randomized, cross-over bioequivalence study of Seratrodast 80mg under fasting condition. Data on file (appears on website on Seretra)
- Hiraga K, Tateno M (1993). "The clinical phase I study of AA-2414, a thromboxane A, receptor antagonist – repeated-dose study at 160 mg once daily for 7 days". Clin Pharmacol. 9 (Suppl. 8): 41–55.
- Hussein Z, Samara E, Locke CS, Orchard MA, Ringham GL, Granneman GR (April 1994). "Characterization of the pharmacokinetics and pharmacodynamics of a new oral thromboxane A2-receptor antagonist AA-2414 in normal subjects: population analysis". Clinical Pharmacology and Therapeutics. 55 (4): 441–50. doi:10.1038/clpt.1994.54. PMID 8162671.
- Samara EE, Qian J, Locke C, Dean R, Killian A, Granneman GR (1996). "Single-dose and steady-state pharmacokinetics of seratrodast in healthy male and female volunteers". Pharm Res. 13 (Suppl. 9).
- Shiraishi M, Kato K, Terao S, Ashida Y, Terashita Z, Kito G (September 1989). "Quinones. 4. Novel eicosanoid antagonists: synthesis and pharmacological evaluation". Journal of Medicinal Chemistry. 32 (9): 2214–21. doi:10.1021/jm00129a030. PMID 2769691.