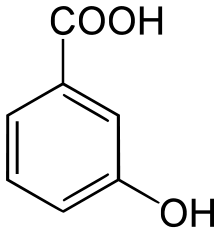
3-Hydroxybenzoic acid
3-Hydroxybenzoic acid is a monohydroxybenzoic acid.
 | |
| Names | |
|---|---|
| IUPAC name
3-Hydroxybenzoic acid | |
| Other names
m-Hydroxybenzoic acid meta-Hydroxybenzoic acid 3-Carboxyphenol m-Salicylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.478 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H6O3 | |
| Molar mass | 138.12 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
3-Hydroxybenzoic acid can be obtained by the alkali fusion of 3-sulfobenzoic acid between 210 – 220°C.[1]
Natural occurrence
3-Hydroxybenzoic acid is a component of castoreum, the exudate from the castor sacs of the mature North American beaver (Castor canadensis) and the European beaver (Castor fiber), used in perfumery.
It can also be formed by a Pseudomonas species from 3-Chlorobenzoic acid.[2]
3-Hydroxybenzoic Acid can be found in the pineapple fruit as well.
gollark: I'll leave the committee to work on it.
gollark: Also no.
gollark: I'm not adding that, either.
gollark: Do you *need* that?
gollark: Maybe this "unidict" thing.
References
- Clarke, M. F.; Owen, L. N. (1950). "434. Alicyclic glycols. Part V. 3-Hydroxymethylcyclohexanol". Journal of the Chemical Society (Resumed): 2108–2115. doi:10.1039/JR9500002108.
- H.W. Johnston, G.G. Briggs and M. Alexander (1972). "Metabolism of 3-chlorobenzoic acid by a pseudomonad". Soil Biology and Biochemistry. 4 (2): 187–190. doi:10.1016/0038-0717(72)90010-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.