Ergosterol
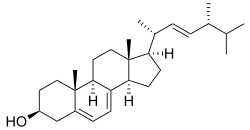
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the enzymes that synthesize it have become important targets for drug discovery. In human nutrition, ergosterol is a provitamin form of vitamin D2; exposure to ultraviolet (UV) light causes a chemical reaction that produces vitamin D2.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
ergosta-5,7,22-trien-3β-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.320 |
| EC Number |
|
| MeSH | Ergosterol |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C28H44O | |
| Molar mass | 396.65 g/mol |
| Melting point | 160 °C (320 °F; 433 K) |
| Boiling point | 250 °C (482 °F; 523 K) |
| -279.6·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Role in fungi
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in fungi, and named for ergot, the common name of members of the fungal genus Claviceps from which ergosterol was first isolated. Ergosterol is a component of yeast and other fungal cell membranes, serving many of the same functions that cholesterol serves in animal cells.[1] Its specificity in higher fungi is thought to be related to the climatic instabilities (highly varying humidity and moisture conditions) encountered by these organisms in their typical ecological niches (plant and animal surfaces, soil). Thus, despite the added energy requirements of ergosterol synthesis (if compared to cholesterol), ergosterol is thought to have evolved as a nearly ubiquitous, evolutionarily advantageous fungal alternative to cholesterol.[2]
Target for antifungal drugs
Because ergosterol is present in cell membranes of fungi, yet absent in those of animals, it is a useful target for antifungal drugs. Ergosterol is also present in the cell membranes of some protists, such as trypanosomes.[3] This is the basis for the use of some antifungals against West African sleeping sickness.
Amphotericin B, an antifungal drug, targets ergosterol. It binds physically to ergosterol within the membrane, thus creating a polar pore in fungal membranes. This causes ions (predominantly potassium and hydrons) and other molecules to leak out, which will kill the cell.[4] Amphotericin B has been replaced by safer agents in most circumstances, but is still used, despite its side effects, for life-threatening fungal or protozoan infections.
Fluconazole, miconazole, itraconazole, clotrimazole, and myclobutanil work in a different way, inhibiting synthesis of ergosterol from lanosterol by interfering with 14α-demethylase.[5] Ergosterol is a smaller molecule than lanosterol; it is synthesized by combining two molecules of farnesyl pyrophosphate, a 15-carbon-long terpenoid, into lanosterol, which has 30 carbons. Then, two methyl groups are removed, making ergosterol. The "azole" class of antifungal agents inhibit the enzyme that performs these demethylation steps in the biosynthetic pathway between lanosterol and ergosterol.[5]
Target for antiprotozoal drugs
Some protozoa, including Trichomonas and Leishmania are inhibited by drugs that target ergosterol synthesis and function[6]
As a vitamin D2 precursor
Ergosterol is a biological precursor of vitamin D2, the chemical name of which is ergocalciferol. Exposure to ultraviolet light causes a photochemical reaction that converts ergosterol to ergocalciferol.[7][8]
This happens naturally to a certain extent, and many mushrooms are irradiated after harvest to increase their vitamin D content. Fungi are also grown industrially so that ergosterol can be extracted and converted to Vitamin D for sale as a dietary supplement and food additive.[8]
Preparations of irradiated ergosterol containing a mixture of previtamin and vitamin D2 were called Viosterol in the 1930s.[9]
Toxicity
Ergosterol powder is an irritant to skin, eyes, and the respiratory tract. Ingestion of large amounts can cause hypercalcemia, which (if prolonged) can lead to calcium salt deposits in the soft tissues and kidneys.[10]
See also
- Mushrooms and vitamin D
References
- Weete JD, Abril M, Blackwell M (2010). "Phylogenetic distribution of fungal sterols". PLOS ONE. 5 (5): e10899. doi:10.1371/journal.pone.0010899. PMC 2878339. PMID 20526375.CS1 maint: multiple names: authors list (link)
- Dupont S, Lemetais G, Ferreira T, Cayot P, Gervais P, Beney L (September 2012). "Ergosterol biosynthesis: a fungal pathway for life on land?". Evolution; International Journal of Organic Evolution. 66 (9): 2961–8. doi:10.1111/j.1558-5646.2012.01667.x. PMID 22946816.
- Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ (February 2003). "Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa". Molecular and Biochemical Parasitology. 126 (2): 129–42. doi:10.1016/S0166-6851(02)00280-3. PMID 12615312.
- Ellis D (February 2002). "Amphotericin B: spectrum and resistance". The Journal of Antimicrobial Chemotherapy. 49 Suppl 1: 7–10. doi:10.1093/jac/49.suppl_1.7. PMID 11801575.
- Lv QZ, Yan L, Jiang YY (August 2016). "The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn". Virulence. 7 (6): 649–59. doi:10.1080/21505594.2016.1188236. PMC 4991322. PMID 27221657.
- Carrillo-Muñoz AJ, Tur-Tur C, Giusiano G, Marcos-Arias C, Eraso E, Jauregizar N, Quindós G (April 2013). "Sertaconazole: an antifungal agent for the topical treatment of superficial candidiasis". Expert Review of Anti-Infective Therapy. 11 (4): 347–58. doi:10.1586/eri.13.17. PMID 23566144.
- Haytowitz, DB Vitamin D in Mushrooms
- Hirsch AL (12 May 2011). "Chapter 6: Industrial Aspects of Vitamin D". In Feldman D, Pike JW, Adam JS (eds.). Vitamin D: Two-Volume Set. Academic Press. ISBN 978-0123819789.
- Science Service (1930). "Viosterol official name for irradiated ergosterol". Journal of Chemical Education. 7 (1): 166. Bibcode:1930JChEd...7..166S. doi:10.1021/ed007p166.
- "Material Safety Data Sheet for Ergosterol". Fisher Scientific.
External links
- "Safety (MSDS) data for ergosterol". Oxford University. 2005. Archived from the original on 2007-10-11. Retrieved 2008-02-10.