Sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide.[11] It is a chaotropic salt.
| |||
 NaI(Tl) scintillators | |||
| Identifiers | |||
|---|---|---|---|
| |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.800 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| NaI | |||
| Molar mass | 149.894[1] | ||
| Appearance | white solid deliquescent[1] | ||
| Odor | odorless | ||
| Density | 3.67 g cm−3[1] | ||
| Melting point | 661 °C (1,222 °F; 934 K) [1] | ||
| Boiling point | 1,304 °C (2,379 °F; 1,577 K) [1] | ||
| 1587 g/L (0 °C) 1842 g/L (25 °C) 2278 g/L (50 °C) 2940 g/L (70 °C) 3020 g/L (100 °C)[2][3] | |||
| Solubility | ethanol, acetone[1] | ||
| Band gap | 5.89 eV[4][5] | ||
| −57×10−6 cm3 mol−1[6] | |||
Refractive index (nD) |
1.93 (300 nm) 1.774 (589 nm) 1.71 (10 µm)[7] | ||
| Structure[8] | |||
| Halite, cF8 | |||
| Fm3m, No. 225 | |||
a = 0.6462 nm | |||
Formula units (Z) |
4 | ||
| Octahedral | |||
| Thermochemistry[9] | |||
Heat capacity (C) |
52.1 J mol−1 K−1 | ||
Std molar entropy (S |
98.5 J mol−1 K−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−287.8 kJ mol−1 | ||
Gibbs free energy (ΔfG˚) |
−286.1 kJ mol−1 | ||
| Hazards | |||
| Main hazards | Irritant, can harm the unborn child | ||
| Safety data sheet | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H315, H319, H400 | ||
| P273, P305+351+338[10] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions |
Sodium fluoride Sodium chloride Sodium bromide Sodium astatide | ||
Other cations |
Lithium iodide Potassium iodide Rubidium iodide Caesium iodide Francium iodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Uses
Food supplement
Sodium iodide, as well as potassium iodide, is commonly used to treat and prevent iodine deficiency. Iodized table salt contains 10 ppm iodide.[11]
Organic synthesis
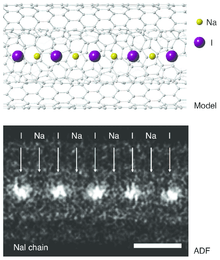
Sodium iodide is used for conversion of alkyl chlorides into alkyl iodides. This method, the Finkelstein reaction,[13] relies on the insolubility of sodium chloride in acetone to drive the reaction:[14]
- R–Cl + NaI → R–I + NaCl
Nuclear medicine
Some radioactive iodide salts of sodium, including Na125I and Na131I, have radiopharmaceutical uses, such as in the treatment of thyroid cancer and hyperthyroidism or as radiolabeling tracers in imaging (see Isotopes of iodine > Radioiodines I-123, I-124, I-125, and I-131 in medicine and biology).
Thallium-doped NaI(Tl) scintillators
Sodium iodide activated with thallium, NaI(Tl), when subjected to ionizing radiation, emits photons (i.e., scintillate) and is used in scintillation detectors, traditionally in nuclear medicine, geophysics, nuclear physics, and environmental measurements. NaI(Tl) is the most widely used scintillation material. The crystals are usually coupled with a photomultiplier tube, in a hermetically sealed assembly, as sodium iodide is hygroscopic. Fine-tuning of some parameters (i.e., radiation hardness, afterglow, transparency) can be achieved by varying the conditions of the crystal growth. Crystals with a higher level of doping are used in X-ray detectors with high spectrometric quality. Sodium iodide can be used both as single crystals and as polycrystals for this purpose. The wavelength of maximum emission is 415 nm.[15]
Solubility data
Sodium iodide exhibits high solubility in some organic solvents, unlike sodium chloride or even bromide:
| Solvent | Solubility of NaI (g NaI/kg of solvent at 25 °C)[16] |
|---|---|
| H2O | 1842 |
| Liquid ammonia | 1620 |
| Liquid sulfur dioxide | 150 |
| Methanol | 625–830 |
| Formic acid | 618 |
| Acetonitrile | 249 |
| Acetone | 504 |
| Formamide | 570–850 |
| Acetamide | 323 (41.5 °C) |
| Dimethylformamide | 37–64 |
| Dichloromethane | 0.09[17] |
Stability
Iodides (including sodium iodide) are detectably oxidized by atmospheric oxygen (O2) to molecular iodine (I2). I2 and I− complex to form the triiodide complex, which has a yellow color, unlike the white color of sodium iodide. Water accelerates the oxidation process, and iodide can also produce I2 by photooxidation, therefore for maximum stability sodium iodide should be stored under dark, low temperature, low humidity conditions.
References
- Haynes, p. 4.86
- Seidell, Atherton (1919). Solubilities of inorganic and organic compounds c. 2. D. Van Nostrand Company. p. 655.
- Haynes, p. 5.171
- Miyata, Takeo (1969). "Exciton Structure of NaI and NaBr". Journal of the Physical Society of Japan. 27 (1): 266. Bibcode:1969JPSJ...27..266M. doi:10.1143/JPSJ.27.266.
- Guizzetti, G.; Nosenzo, L.; Reguzzoni, E. (1977). "Optical properties and electronic structure of alkali halides by thermoreflectivity". Physical Review B. 15 (12): 5921–5926. Bibcode:1977PhRvB..15.5921G. doi:10.1103/PhysRevB.15.5921.
- Haynes, p. 4.130
- Haynes, p. 10.250
- Davey, Wheeler P. (1923). "Precision Measurements of Crystals of the Alkali Halides". Physical Review. 21 (2): 143–161. Bibcode:1923PhRv...21..143D. doi:10.1103/PhysRev.21.143.
- Haynes, p. 5.36
- "Sodium iodide 383112". Sigma Aldrich.
- Lyday, Phyllis A. (2005). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. A14. Weinheim: Wiley-VCH. pp. 382–390. doi:10.1002/14356007.a14_381. ISBN 978-3-527-30673-2.
- Senga, Ryosuke; Suenaga, Kazu (2015). "Single-atom electron energy loss spectroscopy of light elements". Nature Communications. 6: 7943. Bibcode:2015NatCo...6.7943S. doi:10.1038/ncomms8943. PMC 4532884. PMID 26228378.
- Finkelstein, Hank (1910). "Darstellung organischer Jodide aus den entsprechenden Bromiden und Chloriden". Ber. Dtsch. Chem. Ges. (in German). 43 (2): 1528–1532. doi:10.1002/cber.19100430257.
- Streitwieser, Andrew (1956). "Solvolytic Displacement Reactions At Saturated Carbon Atoms". Chemical Reviews. 56 (4): 571–752. doi:10.1021/cr50010a001.
- "Scintillation Materials and Assemblies" (PDF). Saint-Gobain Crystals. 2016. Retrieved June 21, 2017.
- Burgess, John (1978). Metal Ions in Solution. Ellis Horwood Series in Chemical Sciences. New York: Ellis Horwood. ISBN 9780470262931.
- De Namor, Angela F. Danil; Traboulssi, Rafic; Salazar, Franz Fernández; De Acosta, Vilma Dianderas; De Vizcardo, Yboni Fernández; Portugal, Jaime Munoz (1989). "Transfer and partition free energies of 1:1 electrolytes in the water–dichloromethane solvent system at 298.15 K". Journal of the Chemical Society, Faraday Transactions 1. 85 (9): 2705–2712. doi:10.1039/F19898502705.
Cited sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 4.49. ISBN 9781498754293.
External links
| Wikimedia Commons has media related to Sodium iodide. |
- "ICSC 1009 – Sodium Iodide (Anhydrous)". International Chemical Safety Card. April 20, 2005. Retrieved June 21, 2017.
- "Material Safety Data Sheet (MSDS) – Safety data for sodium iodide". ScienceLab.com. May 21, 2013. Retrieved June 21, 2017.
- "Sodium iodide (Oral route, Injection route, Intravenous route)". Drugs.com. 2017. Retrieved June 21, 2017.
- "Safety Data Sheet – Sodium iodide" (PDF). Global Safety Management. January 23, 2015. Retrieved October 16, 2019.