Diacetylene
Diacetylene (also known as butadiyne) is the organic compound with the formula C4H2. It is the simplest compound containing two triple bonds. It is first in the series of polyynes, which are of theoretical but not of practical interest.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Buta-1,3-diyne | |
| Other names
1,3-Butadiyne Biacetylene Butadiyne | |
| Identifiers | |
3D model (JSmol) |
|
| 1236317 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.641 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H2 | |
| Molar mass | 50.060 g·mol−1 |
| Appearance | Gas |
| Boiling point | 10 °C (50 °F; 283 K) |
| Hazards | |
| Main hazards | Highly flammable; Peroxide forming |
| Safety data sheet | External MSDS |
| GHS pictograms | 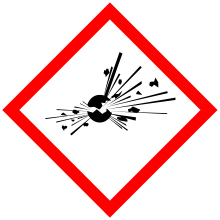  |
| GHS Signal word | Danger |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence
Diacetylene has been identified in the atmosphere of Titan and in the protoplanetary nebula CRL 618 by its characteristic vibrational spectrum. It is proposed to arise by a reaction between acetylene and the ethynyl radical (C2H), which is produced when acetylene undergoes photolysis. This radical can in turn attack the triple bond in acetylene and react efficiently even at low temperatures. Diacetylene has also been detected on the Moon.
Preparation
This compound may be made by the dehydrohalogenation of 1,4-dichloro-2-butyne by potassium hydroxide (in alcoholic medium) at ~70°C:[1]
- ClCH2C≡CCH2Cl + 2 KOH → HC≡C−C≡CH + 2 KCl + 2 H2O
The bis(trimethylsilyl)-protected derivative may be prepared by the Hay coupling of (trimethylsilyl)acetylene:[2]
- 2 Me3Si−C≡CH → Me3Si−C≡C−C≡C−SiMe3
See also
References
- Verkruijsse, H. D.; Brandsma, L. (1991). "A Detailed Procedure for the Preparation of Butadiyne". Synthetic Communications. 21 (5): 657. doi:10.1080/00397919108020833.
- Graham E. Jones, David A. Kendrick, and Andrew B. Holmes (1993). "1,4-Bis(trimethylsilyl)buta-1,3-diyne". Organic Syntheses. doi:10.15227/orgsyn.065.0052.CS1 maint: multiple names: authors list (link); Collective Volume, 8, p. 63
Further reading
- Maretina, Irina A; Trofimov, Boris A (2000). "Diacetylene: a candidate for industrially important reactions". Russian Chemical Reviews. 69 (7): 591. doi:10.1070/RC2000v069n07ABEH000564.