Sodium metavanadate
Sodium metavanadate is the inorganic compound with the formula NaVO3.[1] It is a yellow, water-soluble solid.
 | |
| Names | |
|---|---|
| IUPAC name
Sodium trioxovanadate(V) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ECHA InfoCard | 100.033.869 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NaVO3 | |
| Molar mass | 121.9295 g/mol |
| Appearance | yellow crystalline solid |
| Density | 2.84g/cm3 |
| Melting point | 630 °C (1,166 °F; 903 K) |
| 19.3 g/100 mL (20 °C) 40.8 g/100 mL (80 °C) | |
| Thermochemistry | |
Heat capacity (C) |
97.6 J/mol K |
Std molar entropy (S |
113.8 J/mol K |
Std enthalpy of formation (ΔfH⦵298) |
−1148 kJ/mol |
| Hazards | |
| Main hazards | Toxic, irritant |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
98 mg/kg (rat, oral) |
| Related compounds | |
Other anions |
Sodium orthovanadate |
Other cations |
Ammonium metavanadate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
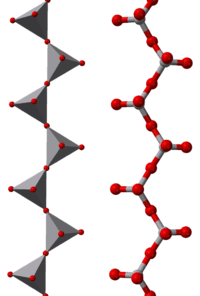
Chain of tetrahedral vanadate [VO4]− units, each sharing two corners
Sodium metavanadate is a common precursor to other vanadates. At low pH it converts to sodium decavanadate. It is also precursor to exotic metalates such as [γ-PV2W10O40]5-, [α-PVW11O40]4-, and [β-PV2W10O40]5-.[2]
Minerals
Sodium metavanadate occurs as two minor minerals, metamunirite (anhydrous) and a dihydrate, munirite. Both are very rare, metamunirite is now known only from vanadium- and uranium-bearing sandstone formations of central-western USA and munirite from Pakistan and South Africa.[3]
gollark: Yes, it is 403ing.
gollark: I see a 403 on my end.
gollark: Check your perms.
gollark: I see your message here however.
gollark: It is done, maybe.
References
- Kato, K.; Takayama, E. (1984). "Das Entwässerungsverhalten des Natriummetavanadatdihydrats und die Kristallstruktur des beta-Natriummetavanadats" [The dehydration activity of sodium metavanadate dihydrate and the crystal structure of β-sodium metavanadate]. Acta Crystallogr. B40 (2): 102–105. doi:10.1107/S0108768184001828.
- Domaille, Peter J. (2007). "Vanadium(V) Substituted Dodecatungstophosphates". Inorganic Syntheses: 96–104. doi:10.1002/9780470132586.ch17. ISBN 9780470132586.
- "Munirite". Mindat.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
