Sodium sulfite
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A pale yellow, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air.[1]
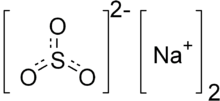 | |||
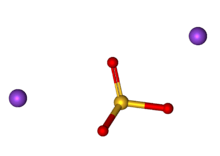 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium sulfite | |||
| Other names
Hypo clear (photography) E221 | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.929 | ||
| EC Number |
| ||
| E number | E221 (preservatives) | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| Na2SO3 | |||
| Molar mass | 126.043 g/mol | ||
| Appearance | White solid | ||
| Odor | Odorless | ||
| Density | 2.633 g/cm3 (anhydrous) 1.561 g/cm3 (heptahydrate) | ||
| Melting point | 33.4 °C (92.1 °F; 306.5 K) (dehydration of heptahydrate) 500 °C (anhydrous) | ||
| Boiling point | Decomposes | ||
| 27.0 g/100 mL water (20 °C) | |||
| Solubility | Soluble in glycerol Insoluble in ammonia, chlorine | ||
| log P | −4 | ||
| Acidity (pKa) | ~9 (heptahydrate) | ||
Refractive index (nD) |
1.565 | ||
| Structure | |||
| Hexagonal (anhydrous) Monoclinic (heptahydrate) | |||
| Hazards | |||
| Safety data sheet | ICSC 1200 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions |
Sodium selenite | ||
Other cations |
Potassium sulfite | ||
Related compounds |
Sodium bisulfite Sodium metabisulfite Sodium sulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Sodium sulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a yellow solid. With more SO2, the solid dissolves to give the disulfite, which crystallizes upon cooling.[1]
- SO2 + 2 NaOH → Na2SO3 + H2O
Sodium sulfite is made industrially by treating sulfur dioxide with a solution of sodium carbonate.[2] The overall reaction is:
- SO2 + Na2CO3 → Na2SO3 + CO2
Applications
Sodium sulfite is primarily used in the pulp and paper industry.[3]
As an oxygen scavenger agent, it is used to treat water being fed to steam boilers to avoid corrosion problems,[4] in the photographic industry, it protects developer solutions from oxidation and (as hypo clear solution) to wash fixer (sodium thiosulfate) from film and photo-paper emulsions
As a reducing agent it is used in the textile industry as a bleaching, desulfurizing, and dechlorinating agent (e.g. in swimming pools). Its reducing properties are exploited in its use as a preservative to prevent dried fruit from discoloring, and for preserving meats.
It is used as a reagent in sulfonation and sulfomethylation agent. It is used in the production of sodium thiosulfate.
Reactions
Heptahydrate
If sodium sulfite is allowed to crystallize from aqueous solution at room temperature or below, it does so as a heptahydrate.[1] The heptahydrate crystals effloresce in warm dry air. Heptahydrate crystals also oxidize in air to form the sulfate. The anhydrous form is much more stable against oxidation by air.[5]
References
- Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. 2: 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
- Weil, Edward D.; Sandler, Stanley R. (1999). "Sulfur Compounds". In Kroschwitz, Jacqueline I. (ed.). Kirk-Othmer Concise Encylclopedia of Chemical Technology (4th ed.). New York: John Wiley & Sons, Inc. p. 1937. ISBN 978-0471419617.
- Barberá, José Jiménez; Metzger, Adolf; Wolf, Manfred (2000). "Sulfites, Thiosulfates, and Dithionitesl Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
- "Pre-boiler and Boiler Corrosion Control | GE Water".
- Merck Index of Chemicals and Drugs, 9th ed. monograph 8451


