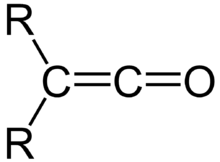
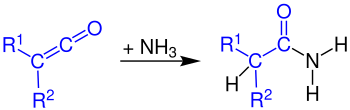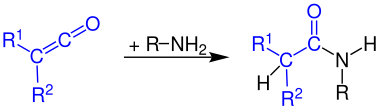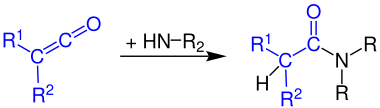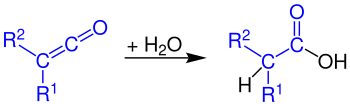Ketene
A ketene is an organic compound of the form R′R″C=C=O, where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule).[1] The name may also refer to the specific compound ethenone H
2C=C=O, the simplest ketene.
Although they are highly useful, most ketenes are unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced.[1]
History
Ketenes were first studied as a class by Hermann Staudinger before 1905.[2]
Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of -chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group).[3]
Preparation
Ethenone, the simplest ketene can be generated by pyrolysis (thermal cracking) of acetone:[4]
- CH3−CO−CH3 → CH2=C=O + CH4
This reaction is called the Schmidlin ketene synthesis.[5][6]
Other ketenes can be prepared from acyl chlorides by an elimination reaction in which HCl is lost:
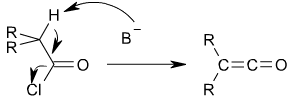 Formation of a ketene from an acyl chloride.
Formation of a ketene from an acyl chloride.
In this reaction, a base, usually triethylamine, removes the acidic proton alpha to the carbonyl group, inducing the formation of the carbon-carbon double bond and the loss of a chloride ion:

Ketenes can also be formed from α-diazoketones by Wolff rearrangement.
Another way to generate ketenes is through flash vacuum thermolysis (FVT) with 2-pyridylamines. Plüg and Wentrup developed a method in 1997 that improved on FVT reactions to produce ketenes with a stable FVT that is moisture insensitive, using mild conditions (480 °C). The N-pyridylamines are prepared via a condensation with R-malonates with N-amino(pyridene) and DCC as the solvent.[7]
A more robust method for preparing ketenes is the carbonylation of metal-carbenes, and in situ reaction of the thus produced highly reactive ketenes with suitable reagents such as imines, amines, or alcohols.[8] This method is an efficient one‐pot tandem protocol of the carbonylation of α‐diazocarbonyl compounds and a variety of N‐tosylhydrazones catalysed by Co(II)–porphyrin metalloradicals leading to the formation of ketenes, which subsequently react with a variety of nucleophiles and imines to form esters, amides and β‐lactams. This system has a broad substrate scope and can be applied to various combinations of carbene precursors, nucleophiles and imines.[9]
Reactions and applications
Due to their cumulated double bonds, ketenes are very reactive.[10]
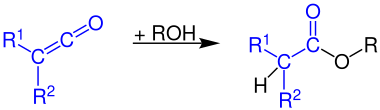
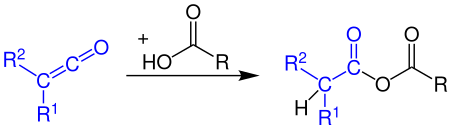
Formation of carboxylic anhydrides
Ketenes react with a carboxylic acids to form carboxylic acid anhydrides:
Formation of carboxylic acid amides
Ketenes react with ammonia to primary amides:
The reaction of ketenes with primary amines produces secondary amides:
Ketenes react with secondary amines to give tertiary amides:
Hydrolysis
By reaction with water, carboxylic acids are formed from ketenes
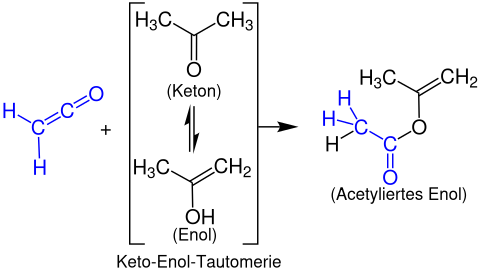
Formation of enol acetates
Enolacetates are formed from ketenes with enolisable carbonyl compounds. The following example shows the reaction of ethenone with acetone to form a propen-2-yl acetate:
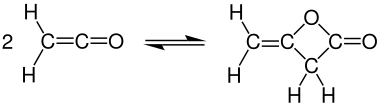
Dimerisation
At room temperature, ketene quickly dimerizes to diketene, but the ketene can be recovered by heating:
[2+2]-Cycloaddition
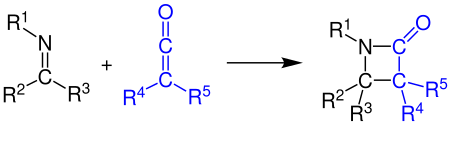
Ketenes can react with alkenes, carbonyl compounds, carbodiimides and imines in a [2+2] cycloaddition. The example shows the synthesis of a β-lactam by the reaction of a ketene with an imine (see Staudinger synthesis):[11][12]
Applications
Ketenes are generally very reactive, and participate in various cycloadditions. One important process is the dimerization to give propiolactones. A specific example is the dimerization of the ketene of stearic acid to afford alkyl ketene dimers, which are widely used in the paper industry.[1] AKD's react with the hydroxyl groups on the celluose via esterification reaction.
They will also undergo [2+2] cycloaddition reactions with electron-rich alkynes to form cyclobutenones, or carbonyl groups to form beta-lactones. With imines beta-lactams are formed. This is the Staudinger synthesis, a facile route to this important class of compounds. With acetone, ketene reacts to give Isopropenyl acetate.[1]
A variety of hydroxylic compounds can add as nucleophiles, forming either enol or ester products. As examples, a water molecule easily adds to ketene to give 1,1-dihydroxyethene and acetic anhydride is produced by the reaction of acetic acid with ketene. Reactions between diols (HO−R−OH) and bis-ketenes (O=C=CH−R′−CH=C=O) yield polyesters with a repeat unit of (−O−R−O−CO−R′−CO).
Ethyl acetoacetate, an important starting material in organic synthesis, can be prepared using a diketene in reaction with ethanol. They directly form ethyl acetoacetate, and the yield is high when carried out under controlled circumstances; this method is therefore used industrially.
See also
References
- Miller R, Abaecherli C, Said A, Jackson B (2001). "Ketenes". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_063. ISBN 978-3527306732.
- Staudinger H (1905). "Ketene, eine neue Körperklasse" [Ketenes, a new class of substances]. Berichte der Deutschen Chemischen Gesellschaft. 38 (2): 1735–1739. doi:10.1002/cber.19050380283.
- Thomas T. Tidwell, The first century of Ketenes (1905-2005): the birth of a family of reactive intermediates, Angewandte Chemie, Int. Edition, Band 44, 2005, S. 5778–5785
- Weygand C (1972). Hilgetag G, Martini A (eds.). Weygand/Hilgetag Preparative Organic Chemistry (4th ed.). New York: John Wiley & Sons, Inc. pp. 1031–1032. ISBN 978-0471937494.
- Hurd CD, Kamm O (1941). "Ketene in Organic Syntheses". Organic Syntheses. Collective Vol. 1. p. 330.
- Schmidlin J, Bergman M (1910). "Darstellung des Ketens aus Aceton" [Preparation of ketene from acetone]. Berichte der Deutschen Chemischen Gesellschaft (in German). 43 (3): 2821–2823. doi:10.1002/cber.19100430340.
- Carsten Plüg ,Hussein Kanaani and Curt Wentrup (12 February 2015). "Ketenes from N-(2-Pyridyl)amides". Australian Journal of Chemistry. 68 (4): 687. doi:10.1071/CH14714.
- Paul ND, Chirila A, Lu H, Zhang XP, de Bruin B (September 2013). "Carbene radicals in cobalt(II)-porphyrin-catalysed carbene carbonylation reactions; a catalytic approach to ketenes". Chemistry. 19 (39): 12953–8. doi:10.1002/chem.201301731. PMC 4351769. PMID 24038393.
- Chirila A, van Vliet KM, Paul ND, de Bruin B (2018). "[Co(MeTAA)] Metalloradical Catalytic Route to Ketenes via Carbonylation of Carbene Radicals" (PDF). European Journal of Inorganic Chemistry. 2018 (20–21): 2251–2258. doi:10.1002/ejic.201800101. ISSN 1099-0682.
- Siegfried Hauptmann (1985), Organische Chemie: mit 65 Tabellen (in German), Leipzig: Deutscher Verlag für Grundstoffindustrie, pp. 410–412, ISBN 3871449024
- Jie Jack Li (2006), Name reactions. A collection of detailed reaction mechanisms (in German) (3 ed.), Berlin: Springer-Verlag, pp. 561-562, doi:10.1007/3-540-30031-7, ISBN 9783540300304
- Hermann Staudinger (1907), "Zur Kenntnis der Ketene. Diphenylketen", Justus Liebigs Annalen der Chemie (in German), John Wiley & Sons, Inc., 356 (1–2), pp. 51–123, doi:10.1002/jlac.19073560106
External links