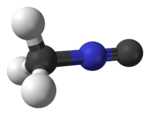
Methyl isocyanide
Methyl isocyanide or isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is isomeric to methyl cyanide (acetonitrile), but its reactivity is very different. Methyl isocyanide is mainly used for making 5-membered heterocyclic rings. The C-N distance in methyl isocyanide is very short, 1.158 Å as is characteristic of isocyanides. [1]
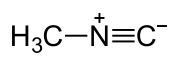 | |
 | |
| Names | |
|---|---|
| IUPAC name
Isocyanomethane | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.008.917 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3N | |
| Molar mass | 41.053 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.69 g/mL |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 59 to 60 °C (138 to 140 °F; 332 to 333 K) |
| Miscible | |
| Hazards | |
EU classification (DSD) (outdated) |
Flammable, harmful |
| R-phrases (outdated) | R11, R20/21/22, R36 |
| S-phrases (outdated) | (S1/2), S16, S36/37 |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds |
acetic acid, acetamide, ethylamine, Acetonitrile |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and uses
Methyl isocyanide was first prepared by A. Gautier by reaction of silver cyanide with methyl iodide.[2][3] The common method for preparing methyl isocyanides is the dehydration of N-methylformamide.[4] Many metal cyanides react with methylating agents to give complexes of methyl isocyanide.[5] This kind of reactivity has been invoked as being relevant to the origin of life.[6]
Methyl isocyanide is useful for the preparation of diverse heterocycles. It is often used to prepare transition metal isocyanide complexes.[7]
References
- Kessler, Myer; Ring, Harold; Trambarulo, Ralph; Gordy, Walter (1950-07-01). "Microwave Spectra and Molecular Structures of Methyl Cyanide and Methyl Isocyanide". Physical Review. American Physical Society (APS). 79 (1): 54–56. Bibcode:1950PhRv...79...54K. doi:10.1103/physrev.79.54. ISSN 0031-899X.
- Gautier, A. (1868). "Ueber eine neue Reihe von Verbindungen, welche mit den Cyanwasserstoffsäure-Aethern isomer sind". Justus Liebigs Annalen der Chemie. 146 (1): 119–124. doi:10.1002/jlac.18681460107.
- Gautier, A. (1869). "Des Nitriles des Acides Gras: Deuxième Partie - Des Carbylamines". Annales de Chimie et de Physique. 17: 203.
- R. E. Schuster, James E. Scott, and Joseph Casanova, Jr (1966). "Methyl isocyanide". Organic Syntheses. 46: 75. doi:10.15227/orgsyn.046.0075.CS1 maint: multiple names: authors list (link)
- Fehlhammer, Wolf P.; Fritz, Marcus. (1993). "Emergence of a CNH and Cyano Complex Based Organometallic Chemistry". Chemical Reviews. 93 (3): 1243–1280. doi:10.1021/cr00019a016.
- Mariani, Angelica; Russell, David; Javelle, Thomas; Sutherland, John (2018). "A Light-Releasable Potentially Prebiotic Nucleotide Activating Agent". Journal of the American Chemical Society. 140 (28): 8657–8661. doi:10.1021/jacs.8b05189. PMC 6152610. PMID 29965757.
- Eckert, H.; Nestl, A.; Ugi, I. (2001). "Methyl isocyanide". Encyclopedia of Reagents for Organic Synthesis, 8 Volume Set. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rm198. ISBN 0471936235.