Dimethyl ether
Dimethyl ether (DME, also known as methoxymethane) is the organic compound with the formula CH3OCH3, simplified to C2H6O. The simplest ether, it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications. It is an isomer of ethanol.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methoxymethane[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | DME | ||
| 1730743 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.696 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Dimethyl+ether | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1033 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H6O | |||
| Molar mass | 46.069 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Ethereal[2] | ||
| Density | 2.1146 kg/m3 (gas, 0 °C, 1013 mbar)[2] 0.735 g/mL (liquid, -25 °C)[2] | ||
| Melting point | −141 °C; −222 °F; 132 K | ||
| Boiling point | −24 °C; −11 °F; 249 K | ||
| 71 g/L (at 20 °C (68 °F)) | |||
| log P | 0.022 | ||
| Vapor pressure | >100 kPa | ||
| -26.3·10−6 cm3/mol | |||
| 1.30 D | |||
| Thermochemistry | |||
Heat capacity (C) |
65.57 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−184.1 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1.4604 MJ mol−1 | ||
| Hazards | |||
| Safety data sheet | See: data page ≥99% Sigma-Aldrich | ||
| GHS pictograms |  | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H220 | ||
| P210, P410+403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −41 °C (−42 °F; 232 K) | ||
| 350 °C (662 °F; 623 K) | |||
| Explosive limits | 27% | ||
| Related compounds | |||
Related ethers |
Diethyl ether | ||
Related compounds |
Ethanol | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
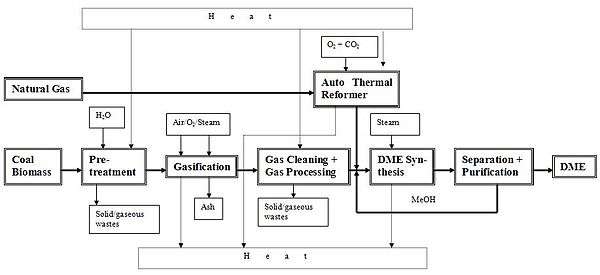
Production
Approximately 50,000 tons were produced in 1985 in Western Europe by dehydration of methanol:[3]
- 2 CH3OH → (CH3)2O + H2O
The required methanol is obtained from synthesis gas (syngas).[4] Other possible improvements call for a dual catalyst system that permits both methanol synthesis and dehydration in the same process unit, with no methanol isolation and purification.[4][5] Both the one-step and two-step processes above are commercially available. The two-step process is relatively simple and start-up costs are relatively low. A one-step liquid-phase process is in development.[4][6]
From biomass
Dimethyl ether is a synthetic second generation biofuel (BioDME), which can be produced from lignocellulosic biomass.[7] The EU is considering BioDME in its potential biofuel mix in 2030;[8] It can also be made from biogas or methane from animal, food, and agricultural waste,[9][10] or even from shale gas or natural gas.[11]
The Volvo Group is the coordinator for the European Community Seventh Framework Programme project BioDME[12][13] where Chemrec's BioDME pilot plant is based on black liquor gasification in Piteå, Sweden.[14]
Applications
The largest use of dimethyl ether is as the feedstock for the production of the methylating agent, dimethyl sulfate, which entails its reaction with sulfur trioxide:
- CH
3OCH
3 + SO
3 → (CH
3)
2SO
4
Dimethyl ether can also be converted into acetic acid using carbonylation technology related to the Monsanto acetic acid process:[3]
- (CH
3)
2O + 2 CO + H2O → 2 CH3CO2H
Laboratory reagent and solvent
Dimethyl ether is a low-temperature solvent and extraction agent, applicable to specialised laboratory procedures. Its usefulness is limited by its low boiling point (−23 °C (−9 °F)), but the same property facilitates its removal from reaction mixtures. Dimethyl ether is the precursor to the useful alkylating agent, trimethyloxonium tetrafluoroborate.[15]
Niche applications
A mixture of dimethyl ether and propane is used in some over-the-counter "freeze spray" products to treat warts, by freezing them.[16][17] In this role, it has supplanted halocarbon compounds (Freon).
Dimethyl ether is also a component of certain high temperature "MAP-plus" blowtorch gas blends, supplanting the use of methyl acetylene and propadiene mixtures.[18]
Dimethyl ether is also used as a propellant in aerosol products. Such products include hair spray, bug spray and some aerosol glue products.
Research
Fuel
A potentially major use of dimethyl ether is as substitute for propane in LPG used as fuel in household and industry.[19]
It is also a promising fuel in diesel engines,[20] and gas turbines. For diesel engines, an advantage is the high cetane number of 55, compared to that of diesel fuel from petroleum, which is 40–53.[21] Only moderate modifications are needed to convert a diesel engine to burn dimethyl ether. The simplicity of this short carbon chain compound leads during combustion to very low emissions of particulate matter. For these reasons as well as being sulfur-free, dimethyl ether meets even the most stringent emission regulations in Europe (EURO5), U.S. (U.S. 2010), and Japan (2009 Japan).[22]
At the European Shell Eco Marathon, an unofficial World Championship for mileage, vehicle running on 100% dimethyl ether drove 589 km/liter (169.8 cm3 /100 km), fuel equivalent to gasoline with a 50 cm3 displacement 2-stroke engine. As well as winning they beat the old standing record of 306 km/liter (326.8 cm3/100 km), set by the same team in 2007.[23]
To study the dimethyl ether for the combustion process a chemical kinetic mechanism[24] is required which can be used for Computational fluid dynamics calculation.
Refrigerant
Dimethyl ether is a refrigerant with ASHRAE refrigerant designation R-E170. It is also used in refrigerant blends with e.g. ammonia, carbon dioxide, butane and propene. Dimethyl ether was the first refrigerant, in 1876, French engineer Charles Tellier bought the ex-Elder-Dempster a 690 tons cargo ship Eboe and fitted a Methyl-ether refrigerating plant of his design. The ship was renamed Le Frigorifique and successfully imported a cargo of refrigerated meat from Argentina. However the machinery could be improved and in 1877 another refrigerated ship called Paraguay with a refrigerating plant improved by Ferdinand Carré was put into service on the South American run.[25]
Safety
Unlike other alkyl ethers, dimethyl ether resists autoxidation. Dimethyl ether is also relatively non-toxic, although it is highly flammable.
Appendix
References
- "CHAPTER P-6. Applications to Specific Classes of Compounds". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 703. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Manfred Müller, Ute Hübsch, “Dimethyl Ether” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a08_541
- "CHEMSYSTEMS.COM" (PDF). www.chemsystems.com. Archived from the original (PDF) on 22 November 2009. Retrieved 1 April 2018.
- P.S. Sai Prasad et al., Fuel Processing Technology, 2008, 89, 1281.
- "Air Products Technology Offerings". airproducts.com. Archived from the original on 12 December 2007. Retrieved 1 April 2018.
- "BioDME". www.biodme.eu. Retrieved 1 April 2018.
- "Biofuels in the European Union, 2006" (PDF). europa.eu. Retrieved 1 April 2018.
- Oberon Fuels Brings Production Units Online, Launching the First North American Fuel-grade DME Facilities
- Associated Gas Utilization via mini GTL
- Ogawa, Takashi; Inoue, Norio; Shikada, Tutomu; Inokoshi, Osamu; Ohno, Yotaro (2004). "Direct Dimethyl Ether (DME) synthesis from natural gas". Natural Gas Conversion VII, Proceedings of the 7th Natural Gas Conversion Symposium. Studies in Surface Science and Catalysis. 147. pp. 379–384. doi:10.1016/S0167-2991(04)80081-8. ISBN 9780444515995.
- "Home | Volvo Group". Archived from the original on 2009-05-25. Retrieved 2011-11-04.
- "Volvo Group - Driving prosperity through transport solutions". www.volvo.com. Retrieved 1 April 2018.
- Chemrec press release September 9, 2010 Archived June 12, 2017, at the Wayback Machine
- T. J. Curphey (1988). "Trimethyloxonium tetrafluoroborate". Organic Syntheses.; Collective Volume, 6, p. 1019
- "A Pharmacist's Guide to OTC Therapy: OTC Treatments for Warts". July 2006. Archived from the original on 2010-06-17. Retrieved 2009-05-02.
- https://www.fda.gov/cdrh/pdf3/K030838.pdf
- http://images.toolbank.com/downloads/cossh/0482.pdf
- "IDA Fact Sheet DME/LPG Blends 2010 v1" (PDF). aboutdme.org. Retrieved 1 April 2018.
- nycomb.se, Nycomb Chemicals company Archived 2008-06-03 at the Wayback Machine
- "Archived copy". Archived from the original on 2007-10-08. Retrieved 2011-11-04.CS1 maint: archived copy as title (link) topsoe.com
- "Archived copy" (PDF). Archived from the original (PDF) on 2009-01-07. Retrieved 2011-11-04.CS1 maint: archived copy as title (link), Conference on the Development and Promotion of Environmentally Friendly Heavy Duty Vehicles such as DME Trucks, Washington DC, March 17, 2006
- "The Danish Ecocar Team - List of achievements". dtu.dk. Archived from the original on 17 October 2009. Retrieved 1 April 2018.
- https://doi.org/10.1016/j.combustflame.2020.04.016
- A history of the frozen meat trade, page 26-28
- http://www.ashrae.org/technology/page/1933#et Archived 2012-01-03 at the Wayback Machine ASHRAE list of refrigerants