Sodium tetrathionate
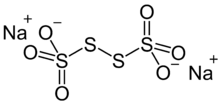
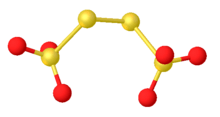
Sodium tetrathionate is a salt of sodium and tetrathionate with the formula Na2S4O6.xH2O. The salt normally is obtained as the dihydrate (x = 2). It is a colorless, water-soluble solid. It is one member of the polythionates, which have the formula [Sn(SO3)2]2-. Other members include trithionite (n = 1), pentathionate (n = 3), hexathionate (n = 4).[1]
 | |
| Names | |
|---|---|
| IUPAC name
Sodium (sulfonatodisulfanyl)sulfonate dihydrate | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.208.917 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na2S4O6 | |
| Molar mass | 306.2665 g/mol (dihydrate) |
| Appearance | white powder |
| Density | 2.1 g/mL (25 ℃) |
| 30.6 g/L (20 ℃) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium tetrathionate is formed by the oxidation of sodium thiosulfate (Na2S2O3), e.g. by the action of iodine:.[2]
- 2 Na2S2O3 + I2 → Na2S4O6 + 2 NaI
The reaction is signaled by the decoloration of iodine. This reaction is the basis of iodometric titrations.
Other methods include the coupling of sodium bisulfite with disulfur dichloride:[1]
- 2 NaHSO3− + S2Cl2 → Na2S4O6 + 2 HCl
The ion has idealizes C2 symmetry, like H2S2. The S-S-S dihedral angle is nearly 90 °. The central S-S distance is 2.115 Å, 0.01 Å longer than the two other S-S distances as well as those distances in most polysulfanes.[3]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Comparative Inorganic Chemistry, Bernard Moody
- P. C. Christidis, P. J. Rentzeperis, C. A. Bolos (1986). "Crystal structure and chirality of sodium tetrathionate dihydrate, Na2S4O6·2H2O". Zeitschrift für Kristallographie. 177: 107-p116. doi:10.1524/zkri.1986.177.1-2.107.CS1 maint: uses authors parameter (link)
