Lithium iodide
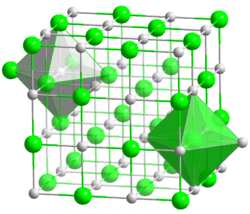
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine.[2] It crystallizes in the NaCl motif.[3] It can participate in various hydrates.[4]
 | |
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.735 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LiI | |
| Molar mass | 133.85 g/mol |
| Appearance | White crystalline solid |
| Density | 4.076 g/cm3 (anhydrous) 3.494 g/cm3 (trihydrate) |
| Melting point | 469 °C (876 °F; 742 K) |
| Boiling point | 1,171 °C (2,140 °F; 1,444 K) |
| 1510 g/L (0 °C) 1670 g/L (25 °C) 4330 g/L (100 °C) [1] | |
| Solubility | soluble in ethanol, propanol, ethanediol, ammonia |
| Solubility in methanol | 3430 g/L (20 °C) |
| Solubility in acetone | 426 g/L (18 °C) |
| −50.0·10−6 cm3/mol | |
Refractive index (nD) |
1.955 |
| Thermochemistry | |
Heat capacity (C) |
0.381 J/g K or 54.4 J/mol K |
Std molar entropy (S |
75.7 J/mol K |
Std enthalpy of formation (ΔfH⦵298) |
-2.02 kJ/g or −270.48 kJ/mol |
Gibbs free energy (ΔfG˚) |
-266.9 kJ/mol |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Lithium fluoride Lithium chloride Lithium bromide Lithium astatide |
Other cations |
Sodium iodide Potassium iodide Rubidium iodide Caesium iodide Francium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
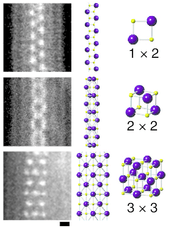
Lithium iodide is used as an electrolyte for high-temperature batteries. It is also used for long-life batteries as required, for example, by artificial pacemakers. The solid is used as a phosphor for neutron detection.[6] It is also used, in a complex with Iodine, in the electrolyte of dye-sensitized solar cells.
In organic synthesis, LiI is useful for cleaving C-O bonds. For example, it can be used to convert methyl esters to carboxylic acids:[7]
- RCO2CH3 + LiI → RCO2Li + CH3I
Similar reactions apply to epoxides and aziridines.
Lithium iodide was used as a radiocontrast agent for CT scans. Its use was discontinued due to renal toxicity. Inorganic iodine solutions suffered from hyperosmolarity and high viscosities. Current iodinated contrast agents are organoiodine compounds.[8]
See also
References
- Patnaik, Pradyot (2002) Handbook of Inorganic Chemicals. McGraw-Hill, ISBN 0-07-049439-8
- "Lithium iodide" (PDF). ESPI Corp. MSDS. Archived from the original (PDF) on 2008-03-09. Retrieved 2005-09-16.
- Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- Wietelmann, Ulrich and Bauer, Richard J. (2005) "Lithium and Lithium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH: Weinheim. doi:10.1002/14356007.a15_393.
- Senga, Ryosuke; Suenaga, Kazu (2015). "Single-atom electron energy loss spectroscopy of light elements". Nature Communications. 6: 7943. doi:10.1038/ncomms8943. PMC 4532884. PMID 26228378.
- Nicholson, K. P.; et al. (1955). "Some lithium iodide phosphors for slow neutron detection". Br. J. Appl. Phys. 6 (3): 104–106. doi:10.1088/0508-3443/6/3/311.
- Charette, André B.; Barbay, J. Kent and He, Wei (2005) "Lithium Iodide" in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons. doi:10.1002/047084289X.rl121.pub2
- Lusic, Hrvoje; Grinstaff, Mark W. (2013). "X-ray-Computed Tomography Contrast Agents". Chemical Reviews. 113 (3): 1641. doi:10.1021/cr200358s. PMC 3878741. PMID 23210836.
External links
| Wikimedia Commons has media related to Lithium iodide. |
- "WebElements – Lithium Iodide". Retrieved 2005-09-16.
- "Composition of Lithium Iodide – NIST". Retrieved 2006-02-03.
