Carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine[1]) is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis.[2] Dialkylcarbodiimides are stable. Some diaryl derivatives tend to convert to dimers and polymers upon standing at room temperature, this mostly occurs with low melting point carbodiimides that are liquid at room temperature.[3] Solid diaryl carbodiimides are stable, but can slowly undergo hydrolysis in the presence of water overtime.
Structure and bonding
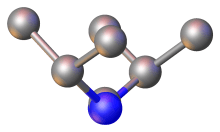
From the perspective of bonding, carbodiimides are isoelectronic with carbon dioxide. Three principal resonance structures describe carbodiimides:
- RN=C=NR ↔ RN+≡C-N−R ↔ RN−-C≡N+R
Relevant to the significance of the polar resonance structures, no carbodiimide has been separated into its optical isomers.[5]
The N=C=N core is relatively linear and the C-N=C angles approach 120″. In the case of C(NCHPh2)2, the central NCN angle is 170° and the C-N=C angles are within 1° of 126°.[4] The C=N distances are short, near 1.20 Å, characteristic of double bonds. Carbodiimides are chiral, possessing C2-symmetry.[6]
The parent compound, methanediimine (HN=C=NH), is a tautomer of cyanamide.
Synthesis
From thioureas and ureas
A classic route to carbodiimides involves dehydrosulfurization of thioureas. A typical reagent is mercuric oxide:[7]
- (R(H)N)2CS + HgO → (RN)2C + HgS + H2O
This reaction can often be conducted as stated, even though carbodiimides react with water. In some cases, a dehydrating agent is added to the reaction mixture.
The dehydration of N,N'-dialkylureas gives carbodiimides:
- (R(H)N)2CO → (RN)2C + H2O
Phosphorus pentoxide[5] and p-Toluenesulfonyl chloride have been used as a dehydrating agents.[8][9]
From isocyanates
Isocyanates convert to carbodiimides with loss of carbon dioxide:[10][3]
- 2 RN=C=O → (RN)2C + CO2
The reaction is catalyzed by phosphine oxides. This reaction is reversible.[7]
Reactions
Compared to other heteroallenes, carbodiimides are very weak electrophiles and only react with nucleophiles in the presence of catalysts, e.g. acids.[11] In this way, guanidines can be prepared.[2] As weak bases, carbodiimides bind to Lewis acids to give adducts.[7]
Moffatt oxidation
Carbodiimides are reagents for the Moffatt oxidation, a protocol for conversion of an alcohol to a carbonyl (ketone or aldehyde) using dimethyl sulfoxide as the oxidizing agent:[12]
- (CH3)2SO + (CyN)2C + R2CHOH → (CH3)2S + (CyNH)2CO + R2C=O
Typically the sulfoxide and diimide are used in excess.[13] The reaction generates dimethyl sulfide and a urea as byproducts.
Coupling agents
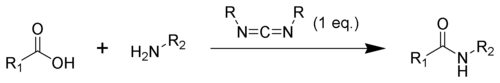
In synthesis, compounds containing the carbodiimide functionality are used as dehydration agents. Specifically they are often used to convert carboxylic acids to amides or esters. Additives, such as N-hydroxybenzotriazole or N-hydroxysuccinimide, are often added to increase yields and decrease side reactions.
Polycarbodiimides can also be used as crosslinkers for aqueous resins, such a polyurethane dispersions or acrylic dispersion. Here the polycarbodiimide reacts with carboxylic acids, which functional groups are often present in such aqueous resins, to form N-acyl urea. The result is that there have formed covalent bonds between the polymer chains, which have thus become crosslinked. [14][15]
Amide formation mechanism
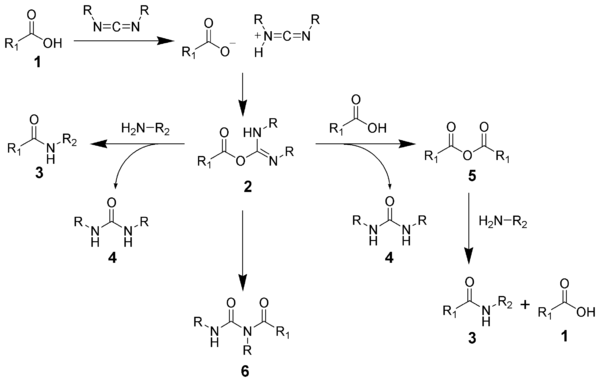
The formation of an amide using a carbodiimide is straightforward, but with several side reactions complicating the subject. The acid 1 will react with the carbodiimide to produce the key intermediate: the O-acylisourea 2, which can be viewed as a carboxylic ester with an activated leaving group. The O-acylisourea will react with amines to give the desired amide 3 and urea 4.
The side reaction of the O-acylisourea 2 produce both desired and undesired products. The O-acylisourea 2 can react with an additional carboxylic acid 1 to give an acid anhydride 5, which can react further to give the desired amide 3. The main undesired reaction pathway involves the rearrangement of the O-acylisourea 2 to the stable N-acylurea 6. The use of solvents with low-dielectric constants such as dichloromethane or chloroform can minimize this side reaction.[16]
Examples
DCC

DCC (acronym for N,N'-dicyclohexylcarbodiimide) was one of the first carbodiimides developed as a reagent. It is widely used for amide and ester formation, especially for solid-phase peptide synthesis. DCC has achieved popularity mainly because of its high yielding amide coupling reactions and the fact that it is quite inexpensive.
However, DCC does have some serious drawbacks, and its use is often avoided for several reasons:
- The byproduct N,N'-dicyclohexylureais mostly removed by filtration, but trace impurities can be difficult to remove. It is incompatible with traditional solid-phase peptide synthesis.
- DCC is a potent allergen, repeated contact with skin increases the probability of sensitization to the compound. Clinical reports of individuals who cannot enter rooms where peptide coupling agents are used have been reported.
For alternative to DCC in coupling see (Coupling Reagents BOP, DCC) at : http://www.biocis.u-psud.fr/IMG/pdf/Coupling_Reagents.pdf
DIC

In contrast to DCC, DIC (acronym for N,N'-diisopropylcarbodiimide) is a liquid. Its hydrolysis product N,N'-diisopropylurea is soluble in organic solvents.
EDC
EDC is a water-soluble carbodiimide reagent used for a wide range of purposes. Apart from uses related to DCC and DIC, it is also used for various biochemical experiments as a crosslinker or chemical probe.
CMCT or CMC
1-cyclohexyl-(2-morpholinoethyl)carbodiimide metho-p-toluene sulfonate is a carbodiimide developed for the chemical probing of RNA structure in biochemistry.
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 375. doi:10.1039/9781849733069-00372. ISBN 978-0-85404-182-4.
The name carbodiimide, for HN=C=NH, is retained but only for general nomenclature; no substitution of any kind is allowed. The systematic name, methanediimine, is the preferred IUPAC name.
- Andrew Williams, Ibrahim T. Ibrahim (1981). "Carbodiimide Chemistry: recent Advances". Chem. Rev. 81 (6): 589–636. doi:10.1021/cr00046a004.CS1 maint: uses authors parameter (link)
- T. W. Campbell, J. J. Monagle (1963). "Diphenylcarbodiimide". Org. Synth. 43: 31. doi:10.15227/orgsyn.043.0031.CS1 maint: uses authors parameter (link)
- Irngartinger, H.; Jäger, H.-U. (1978). "Kristall- und Molekularstrukturen von zwei Carbodiimiden: Bis(diphenylmethyl)carbodiimid und Bis(p-methoxyphenyl)-carbodiimid". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 34 (11): 3262–3265. doi:10.1107/S0567740878010626.
- Henri Ulrich (2008). Chemistry and Technology of Carbodiimides. Wiley-VCH. ISBN 978-0-470-06510-5.
- Vincent, A. T.; Wheatley, P. J. (1972). "Crystal Structure of Bis-p-nitrophenylcarbodiimide, O2N·C6H4·N:C:N·C6H4·NO2". Journal of the Chemical Society, Perkin Transactions 2: 1567–1571. doi:10.1039/P29720001567.
- Frederick Kurzer, K. Douraghi-Zadeh (1967). "Advances in the Chemistry of Carbodiimides". Chem. Rev. 67 (2): ee107–152. doi:10.1021/cr60246a001.CS1 maint: uses authors parameter (link)
- John C. Sheehan, Philip A. Cruickshank (1968). "1-Ethyl-3-(3-Dimethylamino)propylcarbodiimide Hydrochloride and Methiodide". Org. Synth. 48: 83. doi:10.15227/orgsyn.048.0083.CS1 maint: uses authors parameter (link)
- Arnab K. Maity, Skye Fortier, Leonel Griego, Alejandro J. Metta-Magaña (2014). "Synthesis of a "Super Bulky" Guanidinate Possessing an Expandable Coordination Pocket". Inorg. Chem. 53 (15): 8155–8164. doi:10.1021/ic501219q. PMID 25029088.CS1 maint: uses authors parameter (link)
- Monagle, J. J. (1962). "Carbodiimides. III. Conversion of Isocyanates to Carbodiimides. Catalyst Studies". J. Org. Chem. 27 (11): 3851–3855. doi:10.1021/jo01058a022.
- Li, Zhen; Mayer, Robert J.; Ofial, Armin R.; Mayr, Herbert (2020-04-27). "From Carbodiimides to Carbon Dioxide: Quantification of the Electrophilic Reactivities of Heteroallenes". Journal of the American Chemical Society. doi:10.1021/jacs.0c01960.
- Tidwell, T. T. (1990). "Oxidation of Alcohols by Activated Dimethyl Sulfoxide and Related Reactions: An Update". Synthesis. 1990 (10): 857–870. doi:10.1055/s-1990-27036.
- John G. Moffatt (1967). "Cholane-24-al". Org. Synth. 47: 25. doi:10.15227/orgsyn.047.0025.
- Hesselmans, L.C.J.; Derksen, A.J.; van den Goorbergh, J.A.M. (2006). "Polycarbodiimide crosslinkers". Progress in Organic Coatings. 55 (2): 142–148. doi:10.1016/j.porgcoat.2005.08.011. ISSN 0300-9440.
- Posthumus, W.; Derksen, A.J.; van den Goorbergh, J.A.M.; Hesselmans, L.C.J. (2007). "Crosslinking by polycarbodiimides". Progress in Organic Coatings. 58 (2–3): 231–236. doi:10.1016/j.porgcoat.2006.09.031. ISSN 0300-9440.
- Hotan Mojarradi (2010). Coupling of substances containing a primary amine to hyaluronan via carbodiimide-mediated amidation (Thesis). Uppsala Universitet. ISSN 1650-8297.