Sodium cyanoborohydride
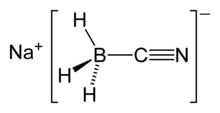
Sodium cyanoborohydride is the chemical compound with the formula NaBH3CN. It is a colourless salt, but commercial samples can appear tan. It is widely used in organic synthesis for the reduction of imines. The salt tolerates aqueous conditions.[2]
 | |
| Names | |
|---|---|
| Other names
Sodium cyanotrihydridoborate | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.043.001 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NaBH3CN | |
| Molar mass | 62.84 gmol−1 |
| Appearance | white to off-white powder, hygroscopic |
| Density | 1.20 g/cm3 |
| Melting point | 241 °C (466 °F; 514 K) decomposes |
| 212 g/100 mL (29 °C) | |
| Solubility | soluble in diglyme, tetrahydrofuran, methanol slightly soluble in methanol insoluble in diethyl ether |
| Hazards | |
| Main hazards | Fatal if swallowed, in contact with skin or if inhaled Contact with acids liberates very toxic gas Contact with water liberates highly flammable gas |
| Safety data sheet | Sigma Aldrich[1] |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H228, H300, H310, H330, H314, H410 |
| P210, P260, P264, P273, P280, P284 | |
| NFPA 704 (fire diamond) | |
Threshold limit value (TLV) |
5 mg/m3 (TWA) |
| Related compounds | |
Other anions |
Sodium borohydride |
Related compounds |
Lithium aluminium hydride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use
Owing to the presence of the electron-withdrawing cyanide substituent, [B(CN)H3]− is far less reducing than is [BH4]−, as found in sodium borohydride.[3] As a mild reducing agent, it is especially used to convert imines to amines.
It is especially favored for reductive aminations, wherein aldehydes or ketones are treated with an amine in the presence of this reagent:
- R2CO + R'NH2 + NaBH3CN + CH3OH → R2CH-NHR' + "NaCH3OBH2CN"
The reagent is typically used in excess. Selectivity is achieved at mildly basic solutions (pH 7-10). The reagent is ideal for reductive aminations ("Borch Reaction").[4] In conjunction with tosylhydrazine, sodium cyanoborohydride is used in the reductive deoxygenation of ketones.[2]
Structure and preparation
The tetrahedral anion BH3(CN)− comprises the salt.
The reagent is invariably purchased, although it can be prepared easily. One method involves combining sodium cyanide and borane. Another route entails treating sodium borohydride with mercury(II) cyanide. The commercial samples can be purified but the yields of the reductive aminations do not improve.[5]
See also
- Sodium triacetoxyborohydride - a milder reductant, but unstable in water
- Sodium borohydride - a stronger, cheaper reductant
References
- Sigma-Aldrich Co., Sodium cyanoborohydride. Retrieved on 2014-11-09.
- Robert O. Hutchins, MaryGail K. Hutchins, Matthew L. Crawley (2007). "Sodium Cyanoborohydride". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rs059.pub2.CS1 maint: uses authors parameter (link)
- Ellen W. Baxter, Allen B. Reitz Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents in Organic Reactions, 2002, John Wiley and Sons. doi:10.1002/0471264180.or059.01
- Richard F. Borch (1988). "Reductive Amination with Sodium Cyanoborohydride: N,N-Dimethylcyclohexylamine". Organic Syntheses.; Collective Volume, 6, p. 499
- Richard F. Borch and Mark D. Bernstein and H. Dupont Durst (1971). "Cyanohydridoborate Anion as a Selective Reducing Agent". J. Am. Chem. Soc. 93 (12): 2897–2904. doi:10.1021/ja00741a013.
