Sodium dithionite
Sodium dithionite (also known as sodium hydrosulfite) is a white crystalline powder with a weak sulfurous odor. Although it is stable in the absence of air, it decomposes in hot water and in acid solutions.
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
D-Ox Hydrolin Reductone Sodium hydrosulfite Sodium sulfoxylate Sulfoxylate Vatrolite Virtex L | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.991 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1384 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na2S2O4 | |
| Molar mass | 174.107 g/mol (anhydrous) 210.146 g/mol (dihydrate) |
| Appearance | white to grayish crystalline powder light-lemon colored flakes |
| Odor | faint sulfur odor |
| Density | 2.38 g/cm3 (anhydrous) 1.58 g/cm3 (dihydrate) |
| Melting point | 52 °C (126 °F; 325 K) |
| Boiling point | Decomposes |
| 18.2 g/100 mL (anhydrous, 20 °C) 21.9 g/100 mL (Dihydrate, 20 °C) | |
| Solubility | slightly soluble in alcohol |
| Hazards | |
EU classification (DSD) (outdated) |
Harmful (Xn) |
| R-phrases (outdated) | R7, R22, R31 |
| S-phrases (outdated) | (S2), S7/8, S26, S28, S43 |
| NFPA 704 (fire diamond) | |
| Flash point | 100 °C (212 °F; 373 K) |
| 200 °C (392 °F; 473 K) | |
| Related compounds | |
Other anions |
Sodium sulfite Sodium sulfate |
Related compounds |
Sodium thiosulfate Sodium bisulfite Sodium metabisulfite Sodium bisulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
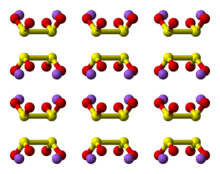
Structure
Raman spectroscopy and single-crystal X-ray diffraction studies reveal that the geometry of the dithionite anion is flexible. The dithionite dianion has C
2 symmetry, with almost eclipsed with a 16° O-S-S-O torsional angle. In the dihydrated form (Na
2S
2O
4·2H
2O), the dithionite anion has a shorter S-S bond length and a gauche 56° O-S-S-O torsional angle.[1]
A weak S-S bond is indicated by the S-S distance of 239 pm. Because this bond is fragile, the dithionite anion dissociates in solution into the [SO2]− radical anion, as has been confirmed by EPR spectroscopy. It is also observed that 35S undergoes rapid exchange between S2O42− and SO2 in neutral or acidic solution, consistent with the weak S-S bond in the anion.[2]
Preparation
Sodium dithionite is produced industrially by reduction of sulfur dioxide. Several methods are employed, including reduction with zinc powder, sodium borohydride, and formate. Approximately 300,000 tons were produced in 1990.[3]
Properties and reactions
Hydrolysis
Sodium dithionite is stable when dry, but aqueous solutions deteriorate due to the following reaction:
- 2 S2O42− + H2O → S2O32− + 2 HSO3−
This behavior is consistent with the instability of dithionous acid. Thus, solutions of sodium dithionite cannot be stored for a long period of time.[2]
Anhydrous sodium dithionite decomposes to sodium sulfate and sulfur dioxide above 90 °C in the air. In absence of air, it decomposes quickly above 150 °C to sodium sulfite, sodium thiosulfate, sulfur dioxide and trace amount of sulfur.
Redox reactions
Sodium dithionite is a reducing agent. At pH=7, the potential is -0.66 V vs NHE. Redox occurs with formation of sulfite:[4]
- S2O42- + 2 H2O → 2 HSO3− + 2 e− + 2 H+
Sodium dithionite reacts with oxygen:
- Na2S2O4 + O2 + H2O → NaHSO4 + NaHSO3
These reactions exhibit complex pH-dependent equilibria involving bisulfite, thiosulfate, and sulfur dioxide.
Applications
Industry
This compound is a water-soluble salt, and can be used as a reducing agent in aqueous solutions. It is used as such in some industrial dyeing processes, primarily those involving sulfur dyes and vat dyes, where an otherwise water-insoluble dye can be reduced into a water-soluble alkali metal salt (e.g. indigo dye).[7] The reduction properties of sodium dithionite also eliminate excess dye, residual oxide, and unintended pigments, thereby improving overall colour quality.
Sodium dithionite can also be used for water treatment, gas purification, cleaning, and stripping. It can also be used in industrial processes as a sulfonating agent or a sodium ion source. In addition to the textile industry, this compound is used in industries concerned with leather, foods, polymers, photography, and many others. Its wide use is attributable to its low toxicity LD50 at 5 g/kg, and hence its wide range of applications. It is also used as decolourising agent in organic reactions.
Biological sciences
Sodium dithionite is often used in physiology experiments as a means of lowering solutions' redox potential (Eo' -0.66 V vs SHE at pH 7).[8] Potassium ferricyanide is usually used as an oxidizing chemical in such experiments (Eo' ~ .436 V at pH 7). In addition, sodium dithionite is often used in soil chemistry experiments to determine the amount of iron that is not incorporated in primary silicate minerals. Hence, iron extracted by sodium dithionite is also referred to as "free iron." The strong affinity of the dithionite ion for bi- and trivalent metal cations (M2+, M3+) allows it to enhance the solubility of iron, and therefore dithionite is a useful chelating agent.
Geosciences
Sodium dithionite has been used in chemical enhanced oil recovery to stabilize polyacrylamide polymers against radical degradation in the presence of iron. It has also been used in environmental applications to propagate a low Eh front in the subsurface in order to reduce components such as chromium.
Photography
It is used in Kodak fogging developer, FD-70. This is used in the second step in processing black and white positive images, for making slides. It is part of the Kodak Direct Positive Film Developing Outfit.[9]
Home
The main use of sodium dithionite at home is as a decoloring agent for white laundry, when it has been accidentally stained by way of a dyed item slipping into the high temperature washing cycle. It is usually available in 5 gram sachets termed hydrosulfite after the antiquated name of the salt.
Laboratory
Aqueous solutions of sodium dithionite were once used to produce "Fieser's solution' for the removal of oxygen from a gas stream.[10] Pyrithione can be prepared in a two-step synthesis from 2-bromopyridine by oxidation to the N-oxide with a suitable peracid followed by substitution using sodium dithionite to introduce the thiol functional group.[11]
See also
References
- Weinrach, J. B.; Meyer, D. R.; Guy, J. T.; Michalski, P. E.; Carter, K. L.; Grubisha, D. S.; Bennett, D. W. (1992). "A structural study of sodium dithionite and its ephemeral dihydrate: A new conformation for the dithionite ion". Journal of Crystallographic and Spectroscopic Research. 22 (3): 291–301. doi:10.1007/BF01199531.
- Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 520. ISBN 978-0-13-175553-6.
- José Jiménez Barberá; Adolf Metzger; Manfred Wolf (15 June 2000). "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry. Wiley Online Library. doi:10.1002/14356007.a25_477. ISBN 978-3527306732.
- Mayhew, S. G. (2008). "The Redox Potential of Dithionite and SO−2 from Equilibrium Reactions with Flavodoxins, Methyl Viologen and Hydrogen plus Hydrogenase". European Journal of Biochemistry. 85 (2): 535–547. doi:10.1111/j.1432-1033.1978.tb12269.x. PMID 648533.
- J. Org. Chem., 1980, 45 (21), pp 4126–4129, http://pubs.acs.org/doi/abs/10.1021/jo01309a011
- "Aldehyde sulfoxylate systemic fungicides". google.com. Archived from the original on 27 April 2018. Retrieved 27 April 2018.
- Božič, Mojca; Kokol, Vanja (2008). "Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes". Dyes and Pigments. 76 (2): 299–309. doi:10.1016/j.dyepig.2006.05.041.
- MAYHEW, Stephen G. (1978). "The Redox Potential of Dithionite and SO-2 from Equilibrium Reactions with Flavodoxins, Methyl Viologen and Hydrogen plus Hydrogenase". European Journal of Biochemistry. 85 (2): 535–547. doi:10.1111/j.1432-1033.1978.tb12269.x. ISSN 0014-2956. PMID 648533.
- "Kodak Direct Positive Film 5246" (PDF). 125px.com. Kodak. Retrieved 6 November 2019.
- Kenneth L. Williamson "Reduction of Indigo: Sodium Hydrosulfite as a Reducing Agent" J. Chem. Educ., 1989, volume 66, p 359. doi:10.1021/ed066p359.2
- Knight, David W.; Hartung, Jens (15 September 2006). "1-Hydroxypyridine-2(1H)-thione". 1-Hydroxypyridine-2(1H)-thione. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rh067.pub2. ISBN 978-0471936237.
External links
- Sodium dithionite - ipcs inchem[1]
- "Sodium dithionite - ipcs inchem" (PDF). www.inchem.org. Berliln, Germany. 2004. Retrieved 15 June 2018.
