Ethinylestradiol sulfonate/norethisterone acetate
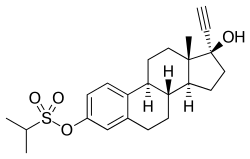
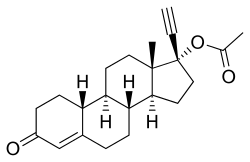
Ethinylestradiol sulfonate/norethisterone acetate (EES/NETA), sold under the brand name Deposiston, is a combination medication of ethinylestradiol sulfonate (EES), an estrogen, and norethisterone acetate (NETA), a progestin, which was used as a combined birth control pill for women.[1] It was formulated as oral tablets and contained 1 mg EES and 5 mg NETA per tablet.[2][3][1] The medication had a long-lasting depot effect and was taken only once per week, for a total of four tablets per cycle.[1][2] It was developed and marketed by Jenapharm and was previously available in Germany.[2][1][3] EES/NETA was introduced for medical use in 1978.[1]
 | |
 | |
| Combination of | |
|---|---|
| Ethinylestradiol sulfonate | Estrogen |
| Norethisterone acetate | Progestogen |
| Clinical data | |
| Trade names | Deposiston |
| Other names | EES/NETA |
| Routes of administration | By mouth |
| Drug class | Estrogen; Progestogen |
References
- Schwarz S, Onken D, Schubert A (July 1999). "The steroid story of Jenapharm: from the late 1940s to the early 1970s". Steroids. 64 (7): 439–45. doi:10.1016/S0039-128X(99)00003-3. PMID 10443899.
6.2. New estrogens. In 1967, Jenapharm, in conjunction with the Academy of Sciences (Kurt Ponsold, Gu¨nter Bruns, and Kurt Schubert in Jena and Hans Schick and Bernard Lu¨cke in Berlin), started a program of searching for new estrogens. [...] orally administered, strongly active estrogens with a depot effect. [...] the second objective was successfully attained. The rationale that an a-branched alkanesulfonic acid ester of ethinyl estradiol with a medium chain length should lead to a depot effect without the danger of active ingredient accumulation on longer usage [15] led in 1978 to the first once-a-week oral contraceptive (DEPOSISTONt), a combination of ethinylestradiol 3-isopropylsulfonate (17) and norethisterone acetate [16]. TURISTERONt, an estrogenic monotherapy with compound 17 that can still justify its position today [17], followed in 1980, as a therapy of prostate cancer. [...]
- Freimut A. Leidenberger (17 April 2013). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 542–. ISBN 978-3-662-08110-5.
- Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 338–. ISBN 978-3-7692-2114-5.
| Progestogens (and progestins) |
| ||||
|---|---|---|---|---|---|
| Antiprogestogens |
| ||||
| |||||
| ER |
| ||||||
|---|---|---|---|---|---|---|---|
| GPER |
| ||||||
| |||||||
| PR |
| ||||||
|---|---|---|---|---|---|---|---|
| mPR (PAQR) |
| ||||||
| |||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.