Cephalosporin
The cephalosporins (sg. /ˌsɛfələˈspɔːrɪn, ˌkɛ-, -loʊ-/[1][2]) are a class of β-lactam antibiotics originally derived from the fungus Acremonium, which was previously known as "Cephalosporium".[3]
| Cephalosporin | |
|---|---|
| Drug class | |
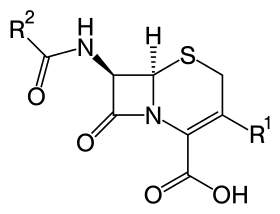 Core structure of the cephalosporins | |
| Class identifiers | |
| Use | Bacterial infection |
| ATC code | J01D |
| Biological target | Penicillin binding proteins |
| Clinical data | |
| Drugs.com | Drug Classes |
| External links | |
| MeSH | D002511 |
| In Wikidata | |
Together with cephamycins, they constitute a subgroup of β-lactam antibiotics called cephems. Cephalosporins were discovered in 1945, and first sold in 1964.[4]
Discovery
The aerobic mold which yielded cephalosporin C was found in the sea near a sewage outfall in Su Siccu, by Cagliari harbour in Sardinia, by the Italian pharmacologist Giuseppe Brotzu in July 1945.[5]
Medical uses
Cephalosporins are indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic. First-generation cephalosporins are active predominantly against Gram-positive bacteria, such as Staphylococcus and Streptococcus.[6] They are therefore used mostly for skin and soft tissue infections and the prevention of hospital-acquired surgical infections.[7] Successive generations of cephalosporins have increased activity against Gram-negative bacteria, albeit often with reduced activity against Gram-positive organisms.
The antibiotic may be used for patients who are allergic to penicillin due to the different β-lactam antibiotic structure. The drug is able to be excreted in the urine.[6]
Side effects
Common adverse drug reactions (ADRs) (≥ 1% of patients) associated with the cephalosporin therapy include: diarrhea, nausea, rash, electrolyte disturbances, and pain and inflammation at injection site. Infrequent ADRs (0.1–1% of patients) include vomiting, headache, dizziness, oral and vaginal candidiasis, pseudomembranous colitis, superinfection, eosinophilia, nephrotoxicity, neutropenia, thrombocytopenia, and fever.
The commonly quoted figure of 10% of patients with allergic hypersensitivity to penicillins and/or carbapenems also having cross-reactivity with cephalosporins originated from a 1975 study looking at the original cephalosporins,[8] and subsequent "safety first" policy meant this was widely quoted and assumed to apply to all members of the group.[9] Hence, it was commonly stated that they are contraindicated in patients with a history of severe, immediate allergic reactions (urticaria, anaphylaxis, interstitial nephritis, etc.) to penicillins, carbapenems, or cephalosporins.[10] This, however, should be viewed in the light of recent epidemiological work suggesting, for many second-generation (or later) cephalosporins, the cross-reactivity rate with penicillin is much lower, having no significantly increased risk of reactivity over the first generation based on the studies examined.[9][11] The British National Formulary previously issued blanket warnings of 10% cross-reactivity, but, since the September 2008 edition, suggests, in the absence of suitable alternatives, oral cefixime or cefuroxime and injectable cefotaxime, ceftazidime, and ceftriaxone can be used with caution, but the use of cefaclor, cefadrocil, cefalexin, and cefradine should be avoided.[12]
Overall, the research shows that all beta lactams have the intrinsic hazard of very serious hazardous reactions in susceptible patients. Only the frequency of these reactions vary, based on the structure. Recent papers have shown that a major feature in determining frequency of immunological reactions is the similarity of the side chains (e.g., first generation cephalosporins are similar to penicillins), and this is the reason the β-lactams are associated with different frequencies of serious reactions (e.g., anaphylaxis).
Several cephalosporins are associated with hypoprothrombinemia and a disulfiram-like reaction with ethanol.[13][14] These include latamoxef (moxalactam), cefmenoxime, cefoperazone, cefamandole, cefmetazole, and cefotetan. This is thought to be due to the N-methylthiotetrazole side-chain of these cephalosporins, which blocks the enzyme vitamin K epoxide reductase (likely causing hypothrombinemia) and aldehyde dehydrogenase (causing alcohol intolerance).[15] Thus, consumption of alcohol after taking Cephalosporin orally or intravenously is contraindicated, and in severe cases can lead to death.
Mechanism of action
Cephalosporins are bactericidal and have the same mode of action as other β-lactam antibiotics (such as penicillins), but are less susceptible to β-lactamases. Cephalosporins disrupt the synthesis of the peptidoglycan layer forming the bacterial cell wall. The peptidoglycan layer is important for cell wall structural integrity. The final transpeptidation step in the synthesis of the peptidoglycan is facilitated by penicillin-binding proteins (PBPs). PBPs bind to the D-Ala-D-Ala at the end of muropeptides (peptidoglycan precursors) to crosslink the peptidoglycan. Beta-lactam antibiotics mimic the D-Ala-D-Ala site, thereby irreversibly inhibiting PBP crosslinking of peptidoglycan.
Resistance
Resistance to cephalosporin antibiotics can involve either reduced affinity of existing PBP components or the acquisition of a supplementary β-lactam-insensitive PBP. Currently, some Citrobacter freundii, Enterobacter cloacae, Neisseria gonorrhoeae, and Escherichia coli strains are resistant to cephalosporins. Some Morganella morganii, Proteus vulgaris, Providencia rettgeri, Pseudomonas aeruginosa, Serratia marcescens and Klebsiella pneumoniae strains have also developed resistance to cephalosporins to varying degrees.[16][17]
Classification
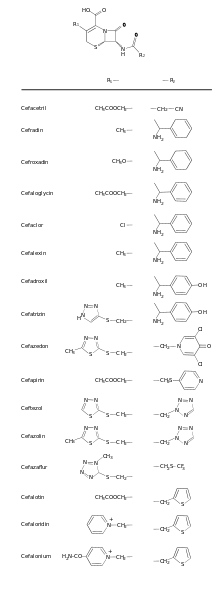
The cephalosporin nucleus can be modified to gain different properties. Cephalosporins are sometimes grouped into "generations" by their antimicrobial properties. The first cephalosporins were designated first-generation cephalosporins, whereas, later, more extended-spectrum cephalosporins were classified as second-generation cephalosporins. Each newer generation has significantly greater Gram-negative antimicrobial properties than the preceding generation, in most cases with decreased activity against Gram-positive organisms. Fourth-generation cephalosporins, however, have true broad-spectrum activity.[18]
The classification of cephalosporins into "generations" is commonly practised, although the exact categorization is often imprecise. For example, the fourth generation of cephalosporins is not recognized as such in Japan. In Japan, cefaclor is classed as a first-generation cephalosporin, though in the United States it is a second-generation one; and cefbuperazone, cefminox, and cefotetan are classed as second-generation cephalosporins. Cefmetazole and cefoxitin are classed as third-generation cephems. Flomoxef and latamoxef are in a new class called oxacephems.[19]
Most first-generation cephalosporins were originally spelled "ceph-" in English-speaking countries. This continues to be the preferred spelling in the United States, Australia, and New Zealand, while European countries (including the United Kingdom) have adopted the International Nonproprietary Names, which are always spelled "cef-". Newer first-generation cephalosporins and all cephalosporins of later generations are spelled "cef-", even in the United States.
Some state that cephalosporins can be divided into five or even six generations, although the usefulness of this organization system is of limited clinical relevance.[20]
Fourth-generation cephalosporins as of March, 2007, were considered to be "a class of highly potent antibiotics that are among medicine's last defenses against several serious human infections" according to the Washington Post.[21]
The mnemonic "LAME" is used to note organisms against which cephalosporins do not have activity:
- Listeria
- Atypicals (including Mycoplasma and Chlamydia)
- MRSA
- Enterococci
Fifth-generation cephalosporins, however, are effective against MRSA.
| Generation | Members | Description |
|---|---|---|
| 1 |
|
Gram-positive: Activity against penicillinase-producing, methicillin-susceptible staphylococci and streptococci (though they are not the drugs of choice for such infections). No activity against methicillin-resistant staphylococci or enterococci.
Gram-negative: Activity against Proteus mirabilis, some Escherichia coli, and Klebsiella pneumoniae ("PEcK"), but have no activity against Bacteroides fragilis, Pseudomonas, Acinetobacter, Enterobacter, indole-positive Proteus, or Serratia. |
| 2 |
Antianaerobe activity: The following cephems are also sometimes grouped with second-generation cephalosporins:
|
Gram-positive: Less than first-generation.
Gram-negative: Greater than first-generation: HEN Haemophilus influenzae, Enterobacter aerogenes and some Neisseria + the PEcK described above. |
| 3 |
Antipseudomonal activity:
These cephems are also sometimes grouped with third-generation cephalosporins:
|
Gram-positive: Some members of this group (in particular, those available in an oral formulation, and those with antipseudomonal activity) have decreased activity against gram-positive organisms.
Activity against staphylococci and streptococci is less with the third-generation compounds than with the first- and second-generation compounds.[23] Gram-negative: Third-generation cephalosporins have a broad spectrum of activity and further increased activity against gram-negative organisms. They may be particularly useful in treating hospital-acquired infections, although increasing levels of extended-spectrum beta-lactamases are reducing the clinical utility of this class of antibiotics. They are also able to penetrate the central nervous system, making them useful against meningitis caused by pneumococci, meningococci, H. influenzae, and susceptible E. coli, Klebsiella, and penicillin-resistant N. gonorrhoeae. Since August 2012, the third-generation cephalosporin, ceftriaxone, is the only recommended treatment for gonorrhea in the United States (in addition to azithromycin or doxycycline for concurrent Chlamydia treatment). Cefixime is no longer recommended as a first-line treatment due to evidence of decreasing susceptibility.[24] |
| 4 |
These cephems are also sometimes grouped with fourth-generation cephalosporins: Note:Cefquinome is not approved for human use. It is for veterinary medicine. |
Gram-positive: They are extended-spectrum agents with similar activity against Gram-positive organisms as first-generation cephalosporins.
Gram-negative: Fourth-generation cephalosporins are zwitterions that can penetrate the outer membrane of Gram-negative bacteria.[25] They also have a greater resistance to β-lactamases than the third-generation cephalosporins. Many can cross the blood–brain barrier and are effective in meningitis. They are also used against Pseudomonas aeruginosa. Cefiderocol has been called a fourth-generation cephalosporin by only one source as of November 2019.[26] |
| 5 | Ceftobiprole has been described as "fifth-generation" cephalosporin,[27][28] though acceptance for this terminology is not universal. Ceftobiprole has anti-pseudomonal activity and appears to be less susceptible to development of resistance. Ceftaroline has also been described as "fifth-generation" cephalosporin, but does not have the activity against Pseudomonas aeruginosa or vancomycin-resistant enterococci that ceftobiprole has.[29] Ceftaroline has activity against MRSA. Ceftolozane is an option for the treatment of complicated intra-abdominal infections and complicated urinary tract infections. It is combined with the β-lactamase inhibitor tazobactam, as multi-drug resistant bacterial infections will generally show resistance to all β-lactam antibiotics unless this enzyme is inhibited.[30][31][32][33][34] | |
| Other: | These cephems have progressed far enough to be named, but have not been assigned to a particular generation:
|
Nitrocefin is a chromogenic cephalosporin substrate, and is used for detection of β-lactamases. |
History
Cephalosporin compounds were first isolated from cultures of Acremonium strictum from a sewer in Sardinia in 1948 by Italian scientist Giuseppe Brotzu.[35] He noticed these cultures produced substances that were effective against Salmonella typhi, the cause of typhoid fever, which had β-lactamase. Guy Newton and Edward Abraham at the Sir William Dunn School of Pathology at the University of Oxford isolated cephalosporin C. The cephalosporin nucleus, 7-aminocephalosporanic acid (7-ACA), was derived from cephalosporin C and proved to be analogous to the penicillin nucleus 6-aminopenicillanic acid (6-APA), but it was not sufficiently potent for clinical use. Modification of the 7-ACA side chains resulted in the development of useful antibiotic agents, and the first agent, cefalotin (cephalothin), was launched by Eli Lilly and Company in 1964.
References
- "cephalosporin". Merriam-Webster Dictionary.
- "cephalosporin - definition of cephalosporin in English from the Oxford dictionary". OxfordDictionaries.com. Retrieved 20 January 2016.
- "cephalosporin" at Dorland's Medical Dictionary
- Oxford Handbook of Infectious Diseases and Microbiology. OUP Oxford. 2009. p. 56. ISBN 9780191039621.
- Tilli Tansey; Lois Reynolds, eds. (2000), Post Penicillin Antibiotics: From acceptance to resistance?, Wellcome Witnesses to Contemporary Medicine, History of Modern Biomedicine Research Group, ISBN 978-1-84129-012-6, Wikidata Q29581637
- "Cephalosporins - Infectious Diseases". Merck Manuals Professional Edition. Retrieved 15 May 2019.
- Pandey, Neelanjana; Cascella, Marco (2020). "Beta Lactam Antibiotics". StatPearls. StatPearls. PMID 31424895.
- Dash, C. H. (1 September 1975). "Penicillin allergy and the cephalosporins". Journal of Antimicrobial Chemotherapy. 1 (suppl 3): 107–118. doi:10.1093/jac/1.suppl_3.107. PMID 1201975.
- Pegler, Scott; Healy, Brendan (10 November 2007). "In patients allergic to penicillin, consider second and third generation cephalosporins for life threatening infections". BMJ. 335 (7627): 991. doi:10.1136/bmj.39372.829676.47. PMC 2072043. PMID 17991982.
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- Pichichero, Michael E (2006). "Cephalosporins can be prescribed safely for penicillin-allergic patients". The Journal of Family Practice. 55 (2): 106–12. PMID 16451776.
- "5.1.2 Cephalosporins and other beta-lactams". British National Formulary (56 ed.). London: BMJ Publishing Group Ltd and Royal Pharmaceutical Society Publishing. September 2008. pp. 295. ISBN 978-0-85369-778-7.
- Kitson, Trevor M. (May 1987). "The effect of cephalosporin antibiotics on alcohol metabolism: A review". Alcohol. 4 (3): 143–148. doi:10.1016/0741-8329(87)90035-8. PMID 3593530.
- Shearer, M. J.; Bechtold, H.; Andrassy, K.; Koderisch, J.; McCarthy, P. T.; Trenk, D.; Jähnchen, E.; Ritz, E. (January 1988). "Mechanism of Cephalosporin-induced Hypoprothrombinemia: Relation to Cephalosporin Side Chain, Vitamin K Metabolism, and Vitamin K Status". The Journal of Clinical Pharmacology. 28 (1): 88–95. doi:10.1002/j.1552-4604.1988.tb03106.x. PMID 3350995.
- Stork CM (2006). "Antibiotics, antifungals, and antivirals". In Nelson LH, Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA (eds.). Goldfrank's toxicologic emergencies. New York: McGraw-Hill. p. 847. ISBN 978-0-07-143763-9.
- "Cephalosporin spectrum of resistance". Retrieved 1 July 2012.
- Sutaria, Dhruvitkumar S.; Moya, Bartolome; Green, Kari B.; Kim, Tae Hwan; Tao, Xun; Jiao, Yuanyuan; Louie, Arnold; Drusano, George L.; Bulitta, Jürgen B. (25 May 2018). "First Penicillin-Binding Protein Occupancy Patterns of β-Lactams and β-Lactamase Inhibitors in Klebsiella pneumoniae". Antimicrobial Agents and Chemotherapy. 62 (6): e00282-18. doi:10.1128/AAC.00282-18. PMC 5971569. PMID 29712652.
- "Cephalosporins - Infectious Diseases - Merck Manuals Professional Edition". Merck Manuals Professional Edition. Retrieved 14 June 2018.
- Narisada, Masayuki; Tsuji, Teruji (1990). "1-Oxacephem Antibiotics". Recent Progress in the Chemical Synthesis of Antibiotics. pp. 705–725. doi:10.1007/978-3-642-75617-7_19. ISBN 978-3-642-75619-1.
- "Case Based Pediatrics Chapter".
- Weiss, Rick (4 March 2007). "FDA Rules override Warnings about Drugs". The Washington Post.
- Jędrzejczyk, Tadeusz. "Internetowa Encyklopedia Leków". leki.med.pl. Archived from the original on 7 October 2007. Retrieved 3 March 2007.
- Scholar, Eric M.; Scholar, Eric Michael; Pratt, William B. (2000). The Antimicrobial Drugs. Oxford University Press. p. 108. ISBN 978-0-19-512528-3.
- Centers for Disease Control and Prevention (10 August 2012). "Update to CDC's Sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections". Morbidity and Mortality Weekly Report. 61 (31): 590–594. PMID 22874837.
- Richard L Sweet; Ronald S. Gibbs (1 March 2009). Infectious Diseases of the Female Genital Tract. Lippincott Williams & Wilkins. pp. 403–. ISBN 978-0-7817-7815-2. Retrieved 8 September 2010.
- "CHEBI:140376 – cefiderocol". ebi.ac.uk. EMBL-EBI. Retrieved 22 November 2019.
- Widmer AF (March 2008). "Ceftobiprole: a new option for treatment of skin and soft-tissue infections due to methicillin-resistant Staphylococcus aureus". Clin. Infect. Dis. 46 (5): 656–658. doi:10.1086/526528. PMID 18225983.
- Kosinski, Mark A.; Joseph, Warren S. (July 2007). "Update on the Treatment of Diabetic Foot Infections". Clinics in Podiatric Medicine and Surgery. 24 (3): 383–396. doi:10.1016/j.cpm.2007.03.009. PMID 17613382.
- Kollef MH (December 2009). "New antimicrobial agents for methicillin-resistant Staphylococcus aureus". Crit Care Resusc. 11 (4): 282–6. PMID 20001879.
- Takeda, S; Nakai, T; Wakai, Y; Ikeda, F; Hatano, K (2007). "In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa". Antimicrobial Agents and Chemotherapy. 51 (3): 826–30. doi:10.1128/AAC.00860-06. PMC 1803152. PMID 17145788.
- Toda, A; Ohki, H; Yamanaka, T; Murano, K; Okuda, S; Kawabata, K; Hatano, K; Matsuda, K; Misumi, K; Itoh, K; Satoh, K; Inoue, S (2008). "Synthesis and SAR of novel parenteral anti-pseudomonal cephalosporins: Discovery of FR264205". Bioorganic & Medicinal Chemistry Letters. 18 (17): 4849–52. doi:10.1016/j.bmcl.2008.07.085. PMID 18701284.
- Sader, H. S.; Rhomberg, P. R.; Farrell, D. J.; Jones, R. N. (2011). "Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes". Antimicrobial Agents and Chemotherapy. 55 (5): 2390–4. doi:10.1128/AAC.01737-10. PMC 3088243. PMID 21321149.
- Craig, W. A.; Andes, D. R. (2013). "In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum β-lactamases, in the thighs of neutropenic mice". Antimicrobial Agents and Chemotherapy. 57 (4): 1577–82. doi:10.1128/AAC.01590-12. PMC 3623364. PMID 23274659.
- Zhanel, G. G.; Chung, P; Adam, H; Zelenitsky, S; Denisuik, A; Schweizer, F; Lagacé-Wiens, P. R.; Rubinstein, E; Gin, A. S.; Walkty, A; Hoban, D. J.; Lynch Jp, 3rd; Karlowsky, J. A. (2014). "Ceftolozane/tazobactam: A novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli". Drugs. 74 (1): 31–51. doi:10.1007/s40265-013-0168-2. PMID 24352909. S2CID 44694926.
- Podolsky, Daniel K. (1998). Cures out of Chaos. CRC Press. ISBN 978-1-4822-2973-8.