Sultamicillin
Sultamicillin, sold under the brand name Unasyn among others, is an oral form of the antibiotic combination (codrug or mutual prodrug) ampicillin/sulbactam. It contains esterified ampicillin and sulbactam.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
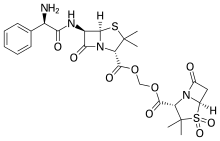
| Formula | C25H30N4O9S2 |
| Molar mass | 594.65 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The pharmacokinetic properties of sultamicillin are improved compared to a combination of ampicillin and sulbactam. Sultamicillin increases the absorption and decreases the chances of diarrhea and dysentery. The inclusion of sulbactam extends ampicillin's spectrum of action to beta-lactamase producing strains of bacteria. Oral sulbactam with parenteral form provides a regimen of continuous sulbactam therapy throughout the treatment, resulting in better clinical results.
It was patented in 1979 and approved for medical use in 1987.[1]
Medical uses
Medical uses for sultamicillin include:
- Skin and soft tissue infections - furuncles, carbuncles, cellulitis, paronychia, impetigo contagiosa, diabetic foot ulcers and abscesses caused by Staphylococcus aureus and Streptococcus pyogenes.
- Upper respiratory tract infections - pharyngitis and tonsillitis caused by S. pyogenes and S. aureus. Acute and chronic sinusitis caused by S. aureus, S. pneumoniae, H. influenzae and S. progenies. otitis media, particularly suppurative otis media, with or without mastoiditis antrum.
- Lower respiratory tract infections - bacterial pneumonias, bronchitis, bronchiectasis caused by S. pneumoniae, H. influenzae, Staphylococcus aureus and S. progenies. Acute exacerbations of COPD.
- Urinary tract infections - pyelonephritis, cystitis caused by Escherichia coli, Proteus mirabilis, Klebsiella, Enterobacter and Staphylococcus aureus.
- Surgical infections - prophylaxis and treatment of surgical site infections, peri-operative prophylaxis in orthopaedic and cardiovascular surgery.
- Gynecological infections - Caused by beta-lactamase producing strains of E. coli and Bacteroides sp. (including B. fragilis).
- Infections of the gastrointestinal tract - Bacterial esophagitis, treatment of H. pylori infections as a part of MDT in ulcer management.
Chemical evaluation
Sultamicillin is a mutual prodrug of ampicillin and sulbactam. Ampicillin, a semi-synthetic orally active broad spectrum antibiotic, is linked via a methylene group with a beta-lactamase inhibitor. Sultamicillin is chemically oxymethyl penicillinate sulfone ester of ampicillin.
Mechanism of action
After absorption, sultamicillin releases ampicillin and sulbactam into the system, so all the antibacterial efficacy of sultamicillin is due to ampicillin and sulbactam. Ampicillin exerts antibacterial activity against sensitive organisms by inhibiting biosynthesis of cell wall mucopeptide where as sulbactam irreversibly inhibits most important beta-lactamases that occur in resistant strains.
References
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 491. ISBN 9783527607495.
Further reading
- Singh GS (January 2004). "Beta-lactams in the new millennium. Part-II: cephems, oxacephems, penams and sulbactam". Mini Reviews in Medicinal Chemistry. 4 (1): 93–109. doi:10.2174/1389557043487547. PMID 14754446.