Aztreonam
Aztreonam, sold under the brand name Azactam among others, is an antibiotic used primarily to treat infections caused by gram-negative bacteria such as Pseudomonas aeruginosa.[1][2] This may include bone infections, endometritis, intra abdominal infections, pneumonia, urinary tract infections, and sepsis.[1] It is given by injection into a vein or muscle or breathed in as a mist.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Azactam, Cayston, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category | |
| Routes of administration | Intravenous, intramuscular, inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IM) 0.1% (by mouth in rats) Unknown (by mouth in humans) |
| Protein binding | 56% |
| Metabolism | Liver (minor %) |
| Elimination half-life | 1.7 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.652 |
| Chemical and physical data | |
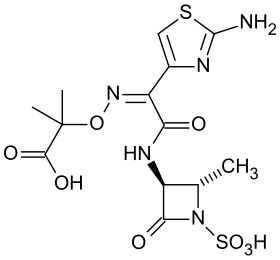
| Formula | C13H17N5O8S2 |
| Molar mass | 435.43 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 227 °C (441 °F) (dec.) |
| |
| |
| (verify) | |
Common side effects when given by injection include pain at the site of injection, vomiting, and rash.[1] Common side effects when inhaled include wheezing, cough, and vomiting.[1] Serious side effects include Clostridium difficile infection and allergic reactions including anaphylaxis.[1] Those who are allergic to other β-lactam have a low rate of allergy to aztreonam.[1] Use in pregnancy appears to be safe.[1] It is in the monobactam family of medications.[1] Aztreonam usually results in bacterial death through blocking their ability to make a cell wall.[1]
Aztreonam was approved for medical use in the United States in 1986.[1] It was removed from the World Health Organization's List of Essential Medicines in 2019.[3][4] It is available as a generic medication.[1] In the UK the injectable form costs the NHS about £28.20 per day while the inhaled form costs about £2,182.00 for a course of treatment.[2] It is a manufactured version of a chemical from the bacterium Chromobacterium violaceum.[5]
Medical uses
Nebulized forms of aztreonam are used to treat infections that are complications of cystic fibrosis and are approved for such use in Europe and the US; they are also used off-label for non-CF bronchiectasis, ventilator-associated pneumonia, chronic obstructive pulmonary disease, mycobacterial disease, and to treat infections in people who have received lung transplants.[6]
Aztreonam has strong activity against susceptible Gram-negative bacteria, including Pseudomonas aeruginosa. It is resistant to some beta-lactamases, but is inactivated by extended-spectrum beta-lactamases.
It has no useful activity against Gram-positive bacteria or anaerobes. It is known to be effective against a wide range of bacteria including Citrobacter, Enterobacter, E. coli, Haemophilus, Klebsiella, Proteus, and Serratia species.[7] The following represents MIC susceptibility data for a few medically significant microorganisms.
- Staphylococcus aureus 8 - >128 μg/ml
- Staphylococcus epidermidis 8 - 32 μg/ml
- Streptococcus pyogenes 8 - ≥128 μg/ml
Synergism between aztreonam and arbekacin or tobramycin against P. aeruginosa has been suggested.[9]
Spectrum of activity
Acinetobacter anitratus, Escherichia coli, Pseudomonas aeruginosa, and Proteus mirabilis are generally susceptible to aztreonam, while some staphylococci, Staphylococcus aureus, Staphylococcus haemolyticus and Xanthomonas maltophilia are resistant to it. Furthermore, Aeromonas hydrophila, Citrobacter diversus, Enterobacter agglomerans, Haemophilus spp. and Streptococcus pyogenes have developed resistance to aztreonam to varying degrees.[10]
Aztreonam is often used in people who are penicillin allergic or who cannot tolerate aminoglycosides.
Administration
Aztreonam is poorly absorbed when given orally, so it must be administered as an intravenous or intramuscular injection (trade name Azactam ), or inhaled (trade name Cayston) using an ultrasonic nebulizer. In the United States, the Food and Drug Administration (FDA) approved the inhalation form on 22 February 2010, for the suppression of P. aeruginosa infections in patients with cystic fibrosis.[11] It received conditional approval for administration in Canada and the European Union in September 2009,[11] and has been fully approved in Australia.[12]
Side effects
Reported side effects include injection site reactions, rash, and rarely toxic epidermal necrolysis. Gastrointestinal side effects generally include diarrhea and nausea and vomiting. There may be drug-induced eosinophilia. Because of the unfused beta-lactam ring unique to aztreonam, there is somewhat lower cross-reactivity between aztreonam and many other beta-lactam antibiotics, and it may be safe to administer aztreonam to many patients with hypersensitivity (allergies) to penicillins and nearly all cephalosporins.[13] However, like other beta lactams, there is a risk of very serious allergic reactions, including anaphylaxis. The aztreonam label directs physicians to be aware of the possibility of these severe adverse reactions. This is more likely if the patient is allergic to a certain cephalosporin known as ceftazidime. Aztreonam exhibits cross-reactivity with this cephalosporin due to a similar side chain. Physicians should evaluate prior allergy history when prescribing this medicine.
Special caution is warranted in patients who are allergic to ceftazidime and are subsequently placed on aztreonam therapy.
Mechanism of action
Aztreonam is similar in action to penicillin. It inhibits synthesis of the bacterial cell wall, by blocking peptidoglycan crosslinking. It has a very high affinity for penicillin-binding protein-3 and mild affinity for penicillin-binding protein-1a. Aztreonam binds the penicillin-binding proteins of Gram-positive and anaerobic bacteria very poorly and is largely ineffective against them.[13] Aztreonam is bactericidal, but less so than some of the cephalosporins.
References
- "Aztreonam". The American Society of Health-System Pharmacists. Retrieved 8 December 2017.
- British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 381. ISBN 9780857111562.
- World Health Organization (2019). Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO Expert Committee on the selection and use of essential medicines. Geneva: World Health Organization. hdl:10665/325773. WHO/MVP/EMP/IAU/2019.05. License: CC BY-NC-SA 3.0 IGO.
- World Health Organization (2019). The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. hdl:10665/330668. ISBN 9789241210300. ISSN 0512-3054. WHO technical report series;1021.
- Yaffe SJ, Aranda JV (2010). Neonatal and Pediatric Pharmacology: Therapeutic Principles in Practice. Lippincott Williams & Wilkins. p. 438. ISBN 9780781795388.
- Quon BS, Goss CH, Ramsey BW (March 2014). "Inhaled antibiotics for lower airway infections". Annals of the American Thoracic Society. 11 (3): 425–34. doi:10.1513/annalsats.201311-395fr. PMC 4028738. PMID 24673698.
- Mosby's Drug Consult 2006 (16th ed.). Mosby, Inc. 2006.
- "Aztreonam Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). toku-e.com. 3 February 2020.
- Kobayashi Y, Uchida H, Kawakami Y (December 1992). "Synergy with aztreonam and arbekacin or tobramycin against Pseudomonas aeruginosa isolated from blood". The Journal of Antimicrobial Chemotherapy. 30 (6): 871–2. doi:10.1093/jac/30.6.871. PMID 1289363.
- "Aztreonam spectrum of bacterial susceptibility and Resistance" (PDF). Retrieved 15 May 2012.
- Larkin C (22 February 2010). "Gilead's Inhaled Antibiotic for Lungs Wins Approval". BusinessWeek. Archived from the original on 2 March 2010. Retrieved 5 March 2010.
- "FDA approves Gilead cystic fibrosis drug Cayston". BusinessWeek. 23 February 2010. Retrieved 5 March 2010.
- AHFS Drug Information 2006 (2006 ed.). American Society of Health-System Pharmacists. 2006.
External links
- "Aztreonam". Drug Information Portal. U.S. National Library of Medicine.