Penem
A penem is a type of unsaturated β-lactam with a sulfur atom in the five-membered ring. Penems do not occur naturally; all are synthetic.[1] Related to penems are carbapenems. Where penems have a sulfur, carbapenems have another carbon.[2]
Structure
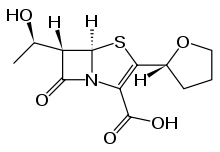
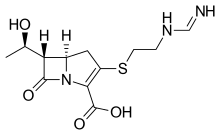
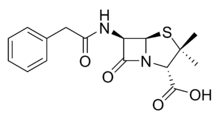
Penem molecules do not occur naturally, and production of penems is an entirely synthetic process.
Five main penem subgroups — thiopenems, oxypenems, aminopenems, alkylpenems, and arylpenems — have been produced and are distinguished by the side chain (at position 2) of the unsaturated five-membered ring. One structurally distinct penem is BRL 42715. This molecule has no substitution at the above position, but has a bulky group attached to the β-lactam ring, and it displays effective inhibition of class C β-lactamases, but no antimicrobial activity.
One possible consequence of these structural differences of penems from other β-lactams may be reduced immunogenicity and immunogenic cross-reactivity.
References
- Richard Wise (1990). "The carbapenems and Penem Antibiotics—a brief review". Antimicrobic Newsletter. 7 (10): 73–78. doi:10.1016/0738-1751(90)90045-E.
- "Medscape.com".
- Milazzo I, Blandino G, Caccamo F, Musumeci R, Nicoletti G, Speciale A (March 2003). "Faropenem, a new oral penem: antibacterial activity against selected anaerobic and fastidious periodontal isolates". Journal of Antimicrobial Chemotherapy. 51 (3): 721–5. doi:10.1093/jac/dkg120. PMID 12615878.
Further reading
- Sasaki A, Goda K, Enomoto M, Sunagawa M (May 1992). "Synthetic studies of carbapenem and penem antibiotics. II. Synthesis of 3-acetyl-2-azetidinones by (2 + 2) cycloaddition of diketene and Schiff bases". Chemical & Pharmaceutical Bulletin. 40 (5): 1094–7. doi:10.1248/cpb.40.1094. PMID 1394625.