Talampicillin
Talampicillin is a beta lactam antibiotic from the penicillin family. It is an acid stable prodrug that was administered orally. It is not approved by the FDA for use in the United States. It should be avoided in Liver diseases
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.194 |
| Chemical and physical data | |
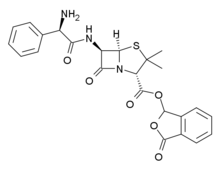
| Formula | C24H23N3O6S |
| Molar mass | 481.52 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Synthesis
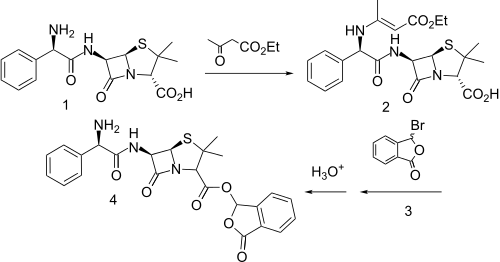
Ampicillin remains the penicillin of choice for many infections because of its oral activity and good potency against Gram-negative bacteria. A number of prodrugs have been examined in attempts to improve upon the pharmacodynamic characteristics, and one of these is talampicillin.
One synthesis involved protecting the primary amino group of ampicillin (1) as the enamine with ethyl acetoacetate (2). THis was then esterified by reaction with 3-bromopthalide (3), and the enamine was carefully hydrolyzed with dilute HCl in acetonitrile to produce talampicillin (4).
References
- I. Isaka, K. Nakano, T. Kashiwagi, A. Koda, H.Horiguchi, H. Matsui, K. Takahashi and M.Murakami, Chem. and Pharm. Bull., 24, 102 (1976).
- J. P. Clayton, M. Cole, S. W. Elson, H. Ferres, J. C. Hanson, L. W. Mizen and R. Sutherland, J.Med. Chem., 19, 1385 (1976).
- H. Ferres, M. P. Clayton, DE 2228012; eidem, US 3860579 (1972, 1975 both to Beecham).
- See also: M. Murakami et al., US 3951954 (1976 to Yamanouchi).