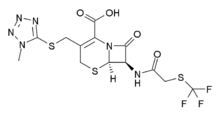
Cefazaflur
Cefazaflur (INN) is a first-generation cephalosporin antibiotic.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H13F3N6O4S3 |
| Molar mass | 470.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Synthesis
Cefazaflur stands out among this group of analogues because it lacks an arylamide C-7 side chain (see cephacetrile for another example).

Cefazaflur synthesis:[1]
Cefazaflur is synthesized by reaction of 3-(1-methyl-1H-tetrazol-5-ylthiomethylene)-7-amino-cephem-4-carboxylic acid (1) with trifluoromethylthioacetyl chloride (2).
gollark: A GUI can be more intuitive in some scenariÖs.
gollark: Also, forcing everyone to spend two years of their life in the military when they may really not like this is quite bad.
gollark: Yes, forcing people to go around killing people and/or helping to is very uncool.
gollark: I meant that ironically™, maybe. There are many Arch install things which are not also a separate distro with its own problems for some reason, and also sinth is using void.
gollark: Indeed.
References
- DeMarinis RM, Boehm JC, Dunn GL, Hoover JR, Uri JV, Guarini JR, et al. (January 1977). "Semisynthetic cephalosporins. Synthesis and structure-activity relationships of analogues with 7-acyl groups derived from 2-(cyanomethylthio)acetic acid or 2-[(2,2,2-trifluoroethyl)thio]acetic acid and their sulfoxides and sulfones". Journal of Medicinal Chemistry. 20 (1): 30–5. doi:10.1021/jm00211a006. PMID 319233.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.