Testosterone undecanoate
Testosterone undecanoate, sold for use by mouth under the brand names Andriol and Jatenzo and for use by injection under the brand names Aveed and Nebido, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels in men.,[4][2][5][6] which includes hormone therapy for transgender men.[7][8][9] It is taken by mouth two to three times per day with food or given by injection into muscle once every 8 to 12 weeks, depending on individual response.[6][10]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /tɛˈstɒstəroʊn ənˈdɛkənoʊeɪt/ teh-STOS-tə-rohn ən-DEK-ə-noh-ayt |
| Trade names | Oral: Andriol, Jatenzo, others IM: Aveed, Nebido, others |
| Other names | TU; Testosterone undecylate; Testosterone 17β-undecanoate; ORG-538; CLR-610 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614041 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth, intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 3–7% Intramuscular: high |
| Protein binding | High (testosterone) |
| Metabolism | Liver |
| Metabolites | Testosterone, undecanoic acid, metabolites of testosterone |
| Elimination half-life | In TSO: 20.9 days (i.m.)[2][3] In CO: 33.9 days (i.m.)[2][3] |
| Excretion | ~90% Urine, 6% feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.025.193 |
| Chemical and physical data | |
| Formula | C30H48O3 |
| Molar mass | 456.711 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects of testosterone undecanoate include symptoms of masculinization like acne, increased hair growth, voice changes, hypertension, elevated liver enzymes, hypertiglyceridemia, and increased sexual desire.[6] The drug is a prodrug of testosterone, the biological ligand of the androgen receptor (AR) and hence is an androgen and anabolic steroid.[11][6] It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization and suitable for androgen replacement therapy.[6] Testosterone undecanoate is a testosterone ester and a prodrug of testosterone in the body.[5][4][2] Because of this, it is considered to be a natural and bioidentical form of testosterone.[12]
Testosterone undecanoate was introduced in China for use by injection and in Europe for use by mouth in the 1970s.[13][14] It became available for use by injection in Europe in the early to mid 2000s and in the United States in 2014.[15][16] A formulation for use by mouth is not currently available in the United States but is pending approval as of 2018.[17] Along with testosterone enanthate, testosterone cypionate, and testosterone propionate, testosterone undecanoate is one of the most widely used testosterone esters.[11][2][6] However, it has advantages over other testosterone esters in that it can be taken by mouth and in that it has a far longer duration when given by injection.[18][4][2][3][6] In addition to its medical use, testosterone undecanoate is used to improve physique and performance.[6] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[6]
Medical uses
Testosterone undecanoate is used in androgen replacement therapy. It is specifically approved only for the treatment of hypogonadism.[19][20][21] As an intramuscular injection, it is administered at a dosage of 1,000 mg once every 12 weeks.[10] Conversely, oral testosterone undecanoate must be taken two or three times a day with food.[10][22]
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosteronea | – | Tablet | 400–800 mg/day (in divided doses) |
| Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg/2–4x day (with meals) | |
| Methyltestosteroneb | Android, Metandren, Testred | Tablet | 10–50 mg/day | |
| Fluoxymesteroneb | Halotestin, Ora-Testryl, Ultandren | Tablet | 5–20 mg/day | |
| Metandienoneb | Dianabol | Tablet | 5–15 mg/day | |
| Mesteroloneb | Proviron | Tablet | 25–150 mg/day | |
| Buccal | Testosterone | Striant | Tablet | 30 mg 2x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 5–25 mg/day | |
| Sublingual | Testosteroneb | Testoral | Tablet | 5–10 mg 1–4x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 10–30 mg/day | |
| Intranasal | Testosterone | Natesto | Nasal spray | 11 mg 3x/day |
| Transdermal | Testosterone | AndroGel, Testim, TestoGel | Gel | 25–125 mg/day |
| Androderm, AndroPatch, TestoPatch | Non-scrotal patch | 2.5–15 mg/day | ||
| Testoderm | Scrotal patch | 4–6 mg/day | ||
| Axiron | Axillary solution | 30–120 mg/day | ||
| Androstanolone (DHT) | Andractim | Gel | 100–250 mg/day | |
| Rectal | Testosterone | Rektandron, Testosteronb | Suppository | 40 mg 2–3x/day |
| Injection (IM or SC) | Testosterone | Andronaq, Sterotate, Virosterone | Aqueous suspension | 10–50 mg 2–3x/week |
| Testosterone propionateb | Testoviron | Oil solution | 10–50 mg 2–3x/week | |
| Testosterone enanthate | Delatestryl | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Xyosted | Auto-injector | 50–100 mg 1x/week | ||
| Testosterone cypionate | Depo-Testosterone | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Testosterone isobutyrate | Agovirin Depot | Aqueous suspension | 50–100 mg 1x/1–2 weeks | |
| Testosterone phenylacetateb | Perandren, Androject | Oil solution | 50–200 mg 1x/3–5 weeks | |
| Mixed testosterone esters | Sustanon 100, Sustanon 250 | Oil solution | 50–250 mg 1x/2–4 weeks | |
| Testosterone undecanoate | Aveed, Nebido | Oil solution | 750–1,000 mg 1x/10–14 weeks | |
| Testosterone buciclatea | – | Aqueous suspension | 600–1,000 mg 1x/12–20 weeks | |
| Implant | Testosterone | Testopel | Pellet | 150–1,200 mg/3–6 months |
| Notes: Men produce about 3 to 11 mg testosterone per day (mean 7 mg/day in young men). Footnotes: a = Never marketed. b = No longer used and/or no longer marketed. Sources: See template. | ||||
| Medication | Brand names | Type | Route | Dosage |
|---|---|---|---|---|
| Testosterone undecanoate | Andriol, Jatenzo | Androgen | Oral | 40–80 mg/2–3x day (with meals) |
| Testosterone | Striant | Androgen | Buccal | 30 mg 2x/day |
| Natesto | Nasal spray | 11 mg 3x/day | ||
| AndroGel, others | Transdermal gel | 25–100 mg/day | ||
| Androderm, others | Transdermal patch | 2.5–10 mg/day | ||
| Axiron | Axillary solution | 30–120 mg/day | ||
| Testopel | Subcutaneous implant | 150–600 mg/3–6 months | ||
| Testosterone enanthate | Delatestryl, others | Androgen | Injection (IM or SC) | 50–100 mg/week or 100–250 mg/2–4 weeks |
| Testosterone cypionate | Depo-Testosterone, others | Androgen | Injection (IM or SC) | 50–100 mg/week or 100–250 mg/2–4 weeks |
| Testosterone isobutyrate | Agovirin Depot | Androgen | Injection (IM or SC) | 50–100 mg/week |
| Mixed testosterone esters | Sustanon 250, others | Androgen | Injection (IM or SC) | 250 mg/2–3 weeks or 500 mg/3–6 weeks |
| Testosterone undecanoate | Aveed, Nebido, others | Androgen | Injection (IM or SC) | 750–1,000 mg/10–14 weeks |
| GnRH analogue | Various | GnRH modulator | Parenteral (various) | Variable |
| Elagolix | Orilissa | GnRH antagonist | Oral | 150 mg/day or 200 mg/twice a day |
| Medroxyprogesterone acetatea | Provera, others | Progestin | Oral | 5–10 mg/day |
| Depo-Provera, others | Injection (IM) | 150 mg/3 months | ||
| Depo-SubQ Provera 104 | Injection (SC) | 104 mg/3 months | ||
| Lynestrenola | Orgametril, others | Progestin | Oral | 5–10 mg/day |
| Finasterideb | Propecia, Proscar | 5α-Reductase inhibitor | Oral | 1 mg/day |
| Dutasterideb | Avodart | 5α-Reductase inhibitor | Oral | 0.5 mg/day |
| Notes: Testes produce 3 to 11 mg testosterone per day (mean 7 mg/day in young men). Footnotes: a = For suppression of menses. b = For prevention/treatment of scalp hair loss. Sources: See template. | ||||
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg 1x/1–2 days |
| Methyltestosterone | Metandren, Estratest | Tablet | 0.5–10 mg/day | |
| Fluoxymesterone | Halotestin | Tablet | 1–2.5 mg 1x/1–2 days | |
| Normethandronea | Ginecoside | Tablet | 5 mg/day | |
| Tibolone | Livial | Tablet | 1.25–2.5 mg/day | |
| Prasterone (DHEA)b | – | Tablet | 10–100 mg/day | |
| Sublingual | Methyltestosterone | Metandren | Tablet | 0.25 mg/day |
| Transdermal | Testosterone | Intrinsa | Patch | 150–300 μg/day |
| AndroGel | Gel, cream | 1–10 mg/day | ||
| Vaginal | Prasterone (DHEA) | Intrarosa | Insert | 6.5 mg/day |
| Injection | Testosterone propionatea | Testoviron | Oil solution | 25 mg 1x/1–2 weeks |
| Testosterone enanthate | Delatestryl, Primodian Depot | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone cypionate | Depo-Testosterone, Depo-Testadiol | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone isobutyratea | Femandren M, Folivirin | Aqueous suspension | 25–50 mg 1x/4–6 weeks | |
| Mixed testosterone esters | Climacterona | Oil solution | 150 mg 1x/4–8 weeks | |
| Omnadren, Sustanon | Oil solution | 50–100 mg 1x/4–6 weeks | ||
| Nandrolone decanoate | Deca-Durabolin | Oil solution | 25–50 mg 1x/6–12 weeks | |
| Prasterone enanthatea | Gynodian Depot | Oil solution | 200 mg 1x/4–6 weeks | |
| Implant | Testosterone | Testopel | Pellet | 50–100 mg 1x/3–6 months |
| Notes: Premenopausal women produce about 230 ± 70 μg testosterone per day (6.4 ± 2.0 mg testosterone per 4 weeks), with a range of 130 to 330 μg per day (3.6–9.2 mg per 4 weeks). Footnotes: a = Mostly discontinued or unavailable. b = Over-the-counter. Sources: See template. | ||||
Side effects
Side effects of testosterone undecanoate include virilization among others.[6]
Anaphylaxis
The Reandron 1000 formulation (Nebido in the United States) contains 1,000 mg of testosterone undecanoate suspended in castor oil with benzyl benzoate for solubilization and as a preservative, and is administered by intramuscular injection. As an excipient in Reandron 1000, benzyl benzoate has been reported as a cause of anaphylaxis (a serious life-threatening allergic reaction) in a case in Australia.[23] Bayer includes this report in information for health professionals and recommends that physicians "should be aware of the potential for serious allergic reactions" to preparations of this type.[24] In Australia, reports to the Adverse Drug Reactions Advisory Committee (ADRAC), which evaluates reports of adverse drug reactions for the Therapeutic Goods Administration (TGA), show several reports of allergic reactions since the anaphylaxis case from 2011.
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Testosterone undecanoate is a prodrug of testosterone and is an androgen and anabolic–androgenic steroid (AAS). That is, it is an agonist of the androgen receptor (AR).
Pharmacokinetics
Testosterone undecanoate has a very long elimination half-life and mean residence time when given as a depot intramuscular injection.[25][2][3] Its elimination half-life is 20.9 days and its mean residence time is 34.9 days in tea seed oil, while its elimination half-life is 33.9 days and its mean residence time is 36.0 days in castor oil.[2][3] These values are substantially longer than those of testosterone enanthate (which, in castor oil, has values of 4.5 days and 8.5 days, respectively).[25] Testosterone undecanoate is administered via intramuscular injection once every three months or so.[10][26]
| Testosterone ester | Form | Route | Tmax | t1/2 | MRT |
|---|---|---|---|---|---|
| Testosterone undecanoate | Oil-filled capsules | Oral | ? | 1.6 hours | 3.7 hours |
| Testosterone propionate | Oil solution | Intramuscular injection | ? | 0.8 days | 1.5 days |
| Testosterone enanthate | Castor oil solution | Intramuscular injection | 10 days | 4.5 days | 8.5 days |
| Testosterone undecanoate | Tea seed oil solution | Intramuscular injection | 13.0 days | 20.9 days | 34.9 days |
| Testosterone undecanoate | Castor oil solution | Intramuscular injection | 11.4 days | 33.9 days | 36.0 days |
| Testosterone buciclatea | Aqueous suspension | Intramuscular injection | 25.8 days | 29.5 days | 60.0 days |
| Notes: Testosterone cypionate has similar pharmacokinetics to TE. Footnotes: a = Never marketed. Sources: See template. | |||||
| Medication | Form | Major brand names | Duration |
|---|---|---|---|
| Testosterone | Aqueous suspension | Andronaq, Sterotate, Virosterone | 2–3 days |
| Testosterone propionate | Oil solution | Androteston, Perandren, Testoviron | 3–4 days |
| Testosterone phenylpropionate | Oil solution | Testolent | 8 days |
| Testosterone isobutyrate | Aqueous suspension | Agovirin Depot, Perandren M | 14 days |
| Mixed testosterone estersa | Oil solution | Triolandren | 10–20 days |
| Mixed testosterone estersb | Oil solution | Testosid Depot | 14–20 days |
| Testosterone enanthate | Oil solution | Delatestryl | 14–28 days |
| Testosterone cypionate | Oil solution | Depovirin | 14–28 days |
| Mixed testosterone estersc | Oil solution | Sustanon 250 | 28 days |
| Testosterone undecanoate | Oil solution | Aveed, Nebido | 100 days |
| Testosterone buciclated | Aqueous suspension | 20 Aet-1, CDB-1781e | 90–120 days |
| Nandrolone phenylpropionate | Oil solution | Durabolin | 10 days |
| Nandrolone decanoate | Oil solution | Deca Durabolin | 21–28 days |
| Methandriol | Aqueous suspension | Notandron, Protandren | 8 days |
| Methandriol bisenanthoyl acetate | Oil solution | Notandron Depot | 16 days |
| Metenolone acetate | Oil solution | Primobolan | 3 days |
| Metenolone enanthate | Oil solution | Primobolan Depot | 14 days |
| Note: All are via i.m. injection. Footnotes: a = TP, TV, and TUe. b = TP and TKL. c = TP, TPP, TiCa, and TD. d = Studied but never marketed. e = Developmental code names. Sources: See template. | |||
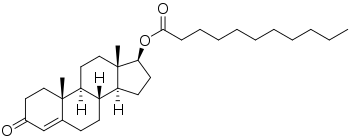
Chemistry
Testosterone undecanoate, or testosterone 17β-undecanoate, is a synthetic androstane steroid and a derivative of testosterone.[27][28] It is an androgen ester; specifically, it is the C17β undecylate (undecanoate) ester of testosterone.[27][28] A related testosterone ester with a similarly very long duration is testosterone buciclate.[4][5]
| Androgen | Structure | Ester | Relative mol. weight | Relative T contentb | Durationc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position | Moiety | Type | Lengtha | Rank | Group | ||||
| Testosterone | – | – | – | – | 1.00 | 1.00 | 11 | Short | |
| Testosterone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.19 | 0.84 | 10 | Short | |
| Testosterone isobutyrate | C17β | Isobutyric acid | Aromatic fatty acid | – (~3) | 1.24 | 0.80 | 9 | Moderate | |
| Testosterone cypionate | C17β | Cyclopentylpropanoic acid | Aromatic fatty acid | – (~6) | 1.43 | 0.70 | 8 | Moderate | |
| Testosterone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 7 | Moderate | |
| Testosterone isocaproate | C17β | Isohexanoic acid | Branched-chain fatty acid | – (~5) | 1.34 | 0.75 | 6 | Moderate | |
| Testosterone caproate | C17β | Hexanoic acid | Straight-chain fatty acid | 6 | 1.35 | 0.75 | 5 | Moderate | |
| Testosterone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.39 | 0.72 | 4 | Moderate | |
| Testosterone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.53 | 0.65 | 3 | Long | |
| Testosterone undecanoate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | 2 | Long | |
| Testosterone buciclated | C17β | Bucyclic acide | Aromatic carboxylic acid | – (~9) | 1.58 | 0.63 | 1 | Long | |
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative testosterone content by weight (i.e., relative androgenic/anabolic potency). c = Duration by intramuscular or subcutaneous injection in oil solution (except TiB and TB, which are in aqueous suspension). d = Never marketed. e = Bucyclic acid = trans-4-Butylcyclohexane-1-carboxylic acid. Sources: See individual articles. | |||||||||
History
In the late 1970s, testosterone undecanoate was introduced for oral use in Europe,[13] although intramuscular testosterone undecanoate had already been in use in China for several years.[14] Intramuscular testosterone undecanoate was not introduced in Europe and the United States until much later, in the early to mid 2000s and 2014, respectively.[15][16] Testosterone undecanoate was approved in the United States after three previous rejections due to safety concerns.[29]
Society and culture
Generic names
Testosterone undecanoate is the generic name of the drug and its USAN and BAN.[27][28][30][31] It is also referred to as testosterone undecylate.[27][28][30][31]
Brand names
Testosterone undecanoate is or has been marketed under a variety of brand names, including Andriol, Androxon, Aveed, Cernos Depot, Jatenzo, Nebido, Nebido-R, Panteston, Reandron 1000, Restandol, and Undestor.[27][28][30][31]
Availability
Intramuscular testosterone undecanoate is available in the United States, Canada, Europe, and elsewhere in the world.[6][32][33] It is approved in over 100 countries worldwide.[32][6] Oral testosterone undecanoate is available in Canada, Europe, Mexico, Asia, and elsewhere but not in the United States.[32][34] Intramuscular testosterone undecanoate is marketed most commonly as Nebido in Canada and Europe and as Aveed in the United States while oral testosterone undecanoate is marketed most commonly as Andriol.[6][32][33]
Legal status
Testosterone undecanoate, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act and a schedule IV controlled substance in Canada under the Controlled Drugs and Substances Act.[35][36]
Research
Non-alcoholic steatohepatitis
In 2013, a phase II clinical trial testing intramuscular testosterone undecanoate for the treatment of non-alcoholic steatohepatitis (NASH) was initiated in the United Kingdom.[37] In the United States in 2018, Lipocine Inc. began investigating the potential of using an oral testosterone undecanoate formulation, known as LPCN-1144, in patients with NASH.[38]
Oral testosterone undecanoate
Recently, two different oral formulations of testosterone undecanoate were developed for the treatment of hypogonadism in the United States.[39][40]
On March 27, 2019, the United States Food and Drug Administration (FDA) approved Jatenzo (testosterone undecanoate), an oral testosterone capsule to treat men with certain forms of hypogonadism. The FDA granted the approval of Jatenzo to Clarus Therapeutics.[41] Clarus has stated it expects Jatenzo to be available in United States pharmacies before the end of 2019,[42] however, it is unclear when Jatenzo will be available for sale as it is currently the subject of a lawsuit alleging patent infringement.[43] The suit was filed by Lipocine Inc., who has also developed an oral testosterone undecanoate drug, Tlando, which (as of May 2019) is under review at the FDA.[44]
References
- "Testosterone Use During Pregnancy". Drugs.com. 20 August 2019. Retrieved 18 March 2020.
- Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (13 January 2010). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 441–446. ISBN 978-3-540-78355-8.
- Behre HM, Abshagen K, Oettel M, Hübler D, Nieschlag E (1999). "Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies". Eur. J. Endocrinol. 140 (5): 414–9. CiteSeerX 10.1.1.503.1752. doi:10.1530/eje.0.1400414. PMID 10229906.
- Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 313–315, 321–322. ISBN 978-1-107-01290-5.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1185–. ISBN 978-0-7817-1750-2.
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 180–182, 331–334. ISBN 978-0-9828280-1-4.
- Irwig MS (2017). "Testosterone therapy for transgender men". Lancet Diabetes Endocrinol. 5 (4): 301–311. doi:10.1016/S2213-8587(16)00036-X. PMID 27084565.
- JW Jacobeit; LJ Gooren; HM Schulte (2007). "Long-acting intramuscular testosterone undecanoate for treatment of female-to-male transgender individuals". The Journal of Sexual Medicine. 4 (5): 1479–84. doi:10.1111/j.1743-6109.2007.00556.x. PMID 17635694.
- JW Jacobeit; LJ Gooren; HM Schulte (2009). "Safety aspects of 36 months of administration of long-acting intramuscular testosterone undecanoate for treatment of female-to-male transgender individuals". European Journal of Endocrinology. 161 (5): 795–8. doi:10.1530/EJE-09-0412. PMID 19749027.
- S. Bertelloni; O. Hiort (28 September 2010). New Concepts for Human Disorders of Sexual Development. S. Karger AG. pp. 256–. ISBN 978-3-8055-9569-8.
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". J. Clin. Endocrinol. Metab. 101 (4): 1318–43. doi:10.1210/jc.2016-1271. PMID 27032319.
- Hoberman J (21 February 2005). Testosterone Dreams: Rejuvenation, Aphrodisia, Doping. University of California Press. pp. 134–. ISBN 978-0-520-93978-3.
- Mundy AR, Fitzpatrick J, Neal DE, George NJ (26 July 2010). The Scientific Basis of Urology. CRC Press. pp. 294–. ISBN 978-1-84184-749-8.
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (11 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 709, 711, 765. ISBN 978-0-323-34157-8.
- Adis R&D Profile (2004). "Testosterone Undecanoate—Schering AG". Drugs. 5 (6): 368–369. doi:10.2165/00126839-200405060-00012. PMID 15563244.
- "Testosterone undecanoate - Clarus Therapeutics - AdisInsight".
- Köhn, Frank-Michael; Schill, Wolf-Bernhard (November 2003). "A new oral testosterone undecanoate formulation". World Journal of Urology. 21 (5): 311–315. doi:10.1007/s00345-003-0372-x. PMID 14579074.
- "Testosterone undecanoate depot injection - AdisInsight".
- "Testosterone undecanoate - Organon - AdisInsight".
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022219s000lbl.pdf
- Jean L. Fourcroy (27 October 2008). Pharmacology, Doping and Sports: A Scientific Guide for Athletes, Coaches, Physicians, Scientists and Administrators. Routledge. pp. 25–. ISBN 978-1-134-08880-5.
- Ong, G. S. Y.; Somerville, C. P.; Jones, T. W.; Walsh, J. P. (2012). "Anaphylaxis Triggered by Benzyl Benzoate in a Preparation of Depot Testosterone Undecanoate". Case Rep Med. 2012: 1–3. doi:10.1155/2012/384054. PMC 3261473. PMID 22272209. 384054.
- "Nebido Monograph – Information for Health Care Professionals". Bayer. 2016. Retrieved 19 October 2016.
- Anita H. Payne; Matthew P. Hardy (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 423–. ISBN 978-1-59745-453-7.
- Yeung SJ, Escalante CP, Gagel RF (2009). Medical Care of Cancer Patients. PMPH-USA. pp. 247–. ISBN 978-1-60795-008-0.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 641–642. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1002–1004. ISBN 978-3-88763-075-1.
- Miriam E. Tucker (March 7, 2014). "FDA Approves Aveed Testosterone Jab, with Restrictions". Medscape. Retrieved December 13, 2016.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- "Testosterone".
- Nieschlag, Eberhard; Nieschlag, Susan (2017). Testosterone. pp. 1–19. doi:10.1007/978-3-319-46086-4_1. ISBN 978-3-319-46084-0.
- "Drug Product Database - Health Canada". Health Canada. March 18, 2010. Archived from the original on November 19, 2016. Retrieved November 13, 2016.
- Arthur P. Arnold; Donald W. Pfaff; Anne M. Etgen; Susan E. Fahrbach, Robert T. Rubin (10 June 2002). Hormones, Brain and Behavior, Five-Volume Set. Academic Press. pp. 20–. ISBN 978-0-12-532104-4.
- Steven B. Karch, MD, FFFLM (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.CS1 maint: multiple names: authors list (link)
- Linda Lane Lilley; Julie S. Snyder; Shelly Rainforth Collins (5 August 2016). Pharmacology for Canadian Health Care Practice. Elsevier Health Sciences. pp. 50–. ISBN 978-1-77172-066-3.
- Testosterone Replacement in Non-alcoholic Steatohepatitis (TEREPINS) (TEREPINS)
- Lipocine Inc. - Clinical Trials
- "Testosterone undecanoate oral twice-daily - Lipocine".
- "Testosterone undecanoate - Clarus Therapeutics".
- FDA approves new oral testosterone capsule for treatment of men with certain forms of hypogonadism

- Clarus Therapeutics Receives U.S. FDA Approval of JATENZO® (Testosterone Undecanoate Capsules for Oral Use) (CIII) for Testosterone Replacement Therapy in Certain Adult Men
- Lipocine Seeks Injunction Against the Marketing of Clarus Therapeutics' JATENZO® for Testosterone Replacement Therapy
- Lipocine Announces TLANDO™ NDA PDUFA Action Date of November 9, 2019
External links
- "Testosterone undecanoate". Drug Information Portal. U.S. National Library of Medicine.