Betalain
Betalains are a class of red and yellow tyrosine-derived pigments found in plants of the Caryophyllales, where they replace anthocyanin pigments. Betalains also occur in some higher order fungi.[1] They are most often noticeable in the petals of flowers, but may color the fruits, leaves, stems, and roots of plants that contain them. They include pigments such as those found in beets.

Description
The name "betalain" comes from the Latin name of the common beet (Beta vulgaris), from which betalains were first extracted. The deep red color of beets, bougainvillea, amaranth, and many cacti results from the presence of betalain pigments.[2] The particular shades of red to purple are distinctive and unlike that of anthocyanin pigments found in most plants.
There are two categories of betalains:[3]
- Betacyanins include the reddish to violet betalain pigments. Among the betacyanins present in plants include betanin, isobetanin, probetanin, and neobetanin.
- Betaxanthins are those betalain pigments which appear yellow to orange. Among the betaxanthins present in plants include vulgaxanthin, miraxanthin, portulaxanthin, and indicaxanthin.
The physiological function of betalains in plants is uncertain, but there is some evidence that they may have fungicidal properties.[4] Additionally, betalains have been found in fluorescent flowers, though their role in these plants is also uncertain.[5]
Chemistry
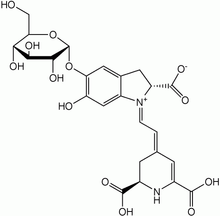
It was once thought that betalains were related to anthocyanins, the reddish pigments found in most plants. Both betalains and anthocyanins are water-soluble pigments found in the vacuoles of plant cells. However, betalains are structurally and chemically unlike anthocyanins and the two have never been found in the same plant together.[6][7] For example, betalains contain nitrogen whereas anthocyanins do not.[2]
It is now known that betalains are aromatic indole derivatives synthesized from tyrosine. They are not related chemically to the anthocyanins and are not even flavonoids.[8] Each betalain is a glycoside, and consists of a sugar and a colored portion. Their synthesis is promoted by light.[3]
The most heavily studied betalain is betanin, also called beetroot red after the fact that it may be extracted from red beet roots. Betanin is a glucoside, and hydrolyzes into the sugar glucose and betanidin.[2] It is used as a food coloring agent, and the color is sensitive to pH. Other betalains known to occur in beets are isobetanin, probetanin, and neobetanin. The color and antioxidant capacity of betanin and indicaxanthin (betaxanthin derived of l-proline) are affected by dielectric microwave heating.[9] Addition of TFE (2,2,2-trifluoroethanol) is reported to improve the hydrolytic stability of some betalains in aqueous solution.[10] Furthermore, a betanin-europium(III) complex has been used to detect calcium dipicolinate in bacterial spores, including Bacillus anthracis and B. cereus.[11]
Other important betacyanins are amaranthine and isoamaranthine, isolated from species of Amaranthus.
Taxonomic significance

Betalain pigments occur only in the Caryophyllales and some Basidiomycota (mushrooms),[12] for instance Hygrophoraceae (waxcaps).[13] Where they occur in plants, they sometimes coexist with anthoxanthins (yellow to orange flavonoids), but never occur in plant species with anthocyanins.[14]
Among the flowering plant order Caryophyllales, most members produce betalains and lack anthocyanins. Of all the families in the Caryophyllales, only the Caryophyllaceae (carnation family) and Molluginaceae produce anthocyanins instead of betalains.[12] The limited distribution of betalains among plants is a synapomorphy for the Caryophyllales, though their production has been lost in two families.
Economic uses

Betanin is commercially used as a natural food dye. It can cause beeturia (red urine) and red feces in some people who are unable to break it down. The interest of the food industry in betalains has grown since they were identified by in vitro methods as antioxidants,[15] which may protect against oxidation of low-density lipoproteins.[16]
Semisynthetic derivatives
Betanin extracted from the red beet[17] was used as starting material for the semisynthesis of an artificial betalainic coumarin, which was applied as a fluorescent probe for the live-cell imaging of Plasmodium-infected erythrocytes.[18]
See also
References
- Strack D, Vogt T, Schliemann W (February 2003). "Recent advances in betalain research". Phytochemistry. 62 (3): 247–69. doi:10.1016/S0031-9422(02)00564-2. PMID 12620337.
- Robinson T (1963). The Organic Constituents of Higher Plants. Minneapolis: Burgess Publishing. p. 292.
- Salisbury FB, Ross CW (1991). Plant Physiology (4th ed.). Belmont, California: Wadsworth Publishing. pp. 325–326. ISBN 978-0-534-15162-1.
- Kimler LM (1975). "Betanin, the red beet pigment, as an antifungal agent". Botanical Society of America, Abstracts of Papers. 36.
- Gandía-Herrero F, García-Carmona F, Escribano J (2005). "Botany: floral fluorescence effect". Nature. 437 (7057): 334. Bibcode:2005Natur.437..334G. doi:10.1038/437334a. PMID 16163341.
- Francis F (1999). Colorants. Egan Press. ISBN 978-1-891127-00-7.
- Stafford HA (1994). "Anthocyanins and betalains: evolution of the mutually exclusive pathways". Plant Science. 101 (2): 91–98. doi:10.1016/0168-9452(94)90244-5. ISSN 0168-9452.
- Raven PH, Evert RF, Eichhorn SE (2004). Biology of Plants (7th ed.). New York: W. H. Freeman and Company. p. 465. ISBN 978-0-7167-1007-3.
- Gonçalves LC, Di Genova BM, Dörr FA, et al. (2013). "Effect of dielectric microwave heating on the color and antiradical capacity of betanin". Journal of Food Engineering. 118 (1): 49–55. doi:10.1016/j.jfoodeng.2013.03.022.
- Bartoloni FH, Gonçalves LC, Rodrigues AC, et al. (2013). "Photophysics and hydrolytic stability of betalains in aqueous trifluoroethanol". Monatshefte für Chemie - Chemical Monthly. 144 (4): 567–571. doi:10.1007/s00706-012-0883-5.
- Gonçalves LC, Da Silva SM, DeRose PC, et al. (2013). "Beetroot-pigment-derived colorimetric sensor for detection of calcium dipicolinate in bacterial spores". PLOS ONE. 8 (9): e73701. Bibcode:2013PLoSO...873701G. doi:10.1371/journal.pone.0073701. PMC 3760816. PMID 24019934.
- Cronquist A (1981). An Integrated System of Classification of Flowering Plants. New York: Columbia University Press. pp. 235–9. ISBN 978-0-231-03880-5.
- Lodge, D. Jean; Padamsee, Mahajabeen; Matheny, P. Brandon; et al. (2013-10-06). "Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales)" (PDF). Fungal Diversity. 64 (1): 1–99. doi:10.1007/s13225-013-0259-0. ISSN 1560-2745.
- Stafford, Helen A. (1994). "Anthocyanins and betalains: evolution of the mutually exclusive pathways (Review)". Plant Science. 101 (2): 91–98. doi:10.1016/0168-9452(94)90244-5. ISSN 0168-9452.
- Escribano J, Pedreño MA, García-Carmona F, Muñoz R (1998). "Characterization of the antiradical activity of betalains from Beta vulgaris L. roots". Phytochem. Anal. 9 (3): 124–7. doi:10.1002/(SICI)1099-1565(199805/06)9:3<124::AID-PCA401>3.0.CO;2-0.
- Tesoriere L, Allegra M, Butera D, Livrea MA (October 2004). "Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: potential health effects of betalains in humans". The American Journal of Clinical Nutrition. 80 (4): 941–5. doi:10.1093/ajcn/80.4.941. PMID 15447903.
- Gonçalves LC, Trassi MA, Lopes NB, et al. (2012). "A comparative study of the purification of betanin". Food Chem. 131: 231–238. doi:10.1016/j.foodchem.2011.08.067.
- Gonçalves LC, Tonelli RR, Bagnaresi P, et al. (2013). Sauer M (ed.). "A nature-inspired betalainic probe for live-cell imaging of Plasmodium-infected erythrocytes". PLOS ONE. 8 (1): e53874. Bibcode:2013PLoSO...853874G. doi:10.1371/journal.pone.0053874. PMC 3547039. PMID 23342028.