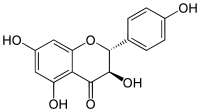
Aromadendrin
Aromadendrin (aromodedrin or dihydrokaempferol) is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | |
| Other names
Aromadedrin Dihydrokaempferol Aromadendrol (+)-Aromadendrin (+)-Dihydrokaempferol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.213.374 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12O6 | |
| Molar mass | 288.255 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Metabolism
The enzyme dihydrokaempferol 4-reductase uses cis-3,4-leucopelargonidin and NADP+ to produce (+)-aromadendrin, NADPH, and H+.
Glycosides
(2R,3R)-trans-Aromadendrin-7-O-beta-D-glucopyranoside-6′′-(4′′′-hydroxy-2′′′-methylene butanoate) is an acylated glucoside of aromadendrin isolated from the stem bark of Afzelia bella[2] (Fabaceae).
Phellamurin is the 8-prenyl 7-glucoside derivative of aromadendrin.
Chemistry
(+)-Leucopelargonidin, (2R,3S,4R)-3,4,5,7,4'-pentahydroxyflavan, can be synthesized from (+)-aromadendrin by sodium borohydride reduction.[3]
gollark: It is not.
gollark: Apparently it could end up (maybe sometimes) being faster because of not having to do context switches.
gollark: There is that neat bare-metal WASM interpreter thing now.
gollark: Anyway, while you can pretty easily verify that "person/address X agreed to transfer money to person/address Y" - just have them sign some sort of transaction object thingy saying so - it's much harder to actually establish a canonical list and ordering of transactions, decide who has coins, etc.
gollark: I believe there are ways to resolve it, somehow.
References
- V. I. Lutskii, A. S. Gromova and N. A. Tyukavkina (1971). "Aromadendrin, apigenin, and kaempferol from the wood of Pinus sibirica". Chemistry of Natural Compounds. 7 (2): 197. doi:10.1007/BF00568701.
- Binutu, OA; Cordell, GA (2001). "Constituents of Afzelia bella stem bark". Phytochemistry. 56 (8): 827–30. doi:10.1016/S0031-9422(01)00006-1. PMID 11324912.
- Heller, Werner; Britsch, Lothar; Forkmann, Gert; Grisebach, Hans (1985). "Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br". Planta. 163 (2): 191. doi:10.1007/BF00393505. PMID 24249337.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.