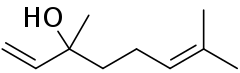
Linalool
Linalool (/lɪˈnæloʊɒl, laɪ-, -loʊoʊl, -ˈluːl/)[1] refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants.[2] These have multiple commercial applications, the majority of which are based on its pleasant scent (floral, with a touch of spiciness).[2]
 | |
-Linalool_molecule_ball.png) | |
| Names | |
|---|---|
| Preferred IUPAC name
3,7-Dimethyl-1,6-octadien-3-ol | |
| Other names
3,7-Dimethylocta-1,6-dien-3-ol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.032 |
| KEGG | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.253 g·mol−1 |
| Density | 0.858 to 0.868 g/cm3 |
| Melting point | < −20 °C (−4 °F; 253 K) |
| Boiling point | 198 to 199 °C (388 to 390 °F; 471 to 472 K) |
| 1.589 g/l | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 55 °C (131 °F; 328 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The word linalool is based on linaloe (a type of wood) and the suffix -ol.[3] It has other names such as β-linalool, linalyl alcohol, linaloyl oxide, allo-ocimenol, and 3,7-dimethyl-1,6-octadien-3-ol.[2]
Nature
Over 200 species of plants produce linalool, mainly from the families Lamiaceae (mint and other herbs), Lauraceae (laurels, cinnamon, rosewood), and Rutaceae (citrus fruits), but also birch trees and other plants, from tropical to boreal climate zones, including fungi.[2]
Enantiomers
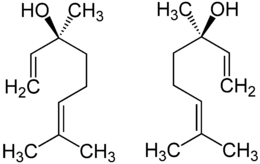
Linalool has a stereogenic center at C3 and therefore there are two stereoisomers: (R)-(–)-linalool is also known as licareol and (S)-(+)-linalool is also known as coriandrol.
Both enantiomeric forms are found in nature: (S)-linalool is found, for example, as a major constituent of the essential oils of coriander (Coriandrum sativum L.), cymbopogon (Cymbopogon martini var. martinii), and sweet orange (Citrus sinensis) flowers. (R)-linalool is present in lavender (Lavandula officinalis), bay laurel (Laurus nobilis), and sweet basil (Ocimum basilicum), among others.
Each enantiomer evokes different neural responses in humans, so are classified as possessing distinct scents. (S)-(+)-Linalool is perceived as sweet, floral, petitgrain-like (odor threshold 7.4 ppb) and the (R)-form as more woody and lavender-like (odor threshold 0.8 ppb).
Biosynthesis
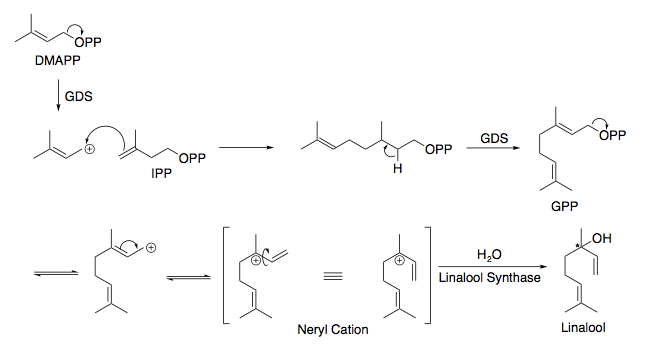
In higher plants, linalool, is an acyclic monoterpenoid. Like the majority of monoterpenes, it starts at the condensation of dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) to form geranyl pyrophosphate (GPP).[4] With the aid of linalool synthase (LIS), water attacks to form the chiral center.[5][4]LIS appears to show a limonene synthase-type catalysis through a simplified "metal-cofactor-binding domain [where the majority] of the residues involved in substrate...binding [are] in the C-terminal part of the protein" suggesting stereoselectivity and the reasoning behind why some plants have varying levels of each enantiomer.[6][7]
Uses
Linalool is used as a scent in 60% to 80% of perfumed hygiene products and cleaning agents including soaps, detergents, shampoos, and lotions.[8]
It is also used as a chemical intermediate. One common downstream product of linalool is vitamin E.
In addition, linalool is used by pest professionals as a flea, fruit fly, and cockroach insecticide. It can also be used as a method of pest control for codling moths. Linalool creates a synergistic effect with the codling moth's pheromone called codlemone, which increases attraction of males.[9]
Linalool is used in some mosquito-repellent products;[10] however, the EPA notes that "a preliminary screen of labels for products containing [l]inalool (as the sole active ingredient) indicates that efficacy data on file with the Agency may not support certain claims to repel mosquitos."[11]
Plants that contain linalool
Safety and potential toxicity
Linalool can be absorbed by inhalation of its aerosol and by oral intake or skin absorption, potentially causing irritation, pain and allergic reactions.[2][17] Some 7% of people undergoing patch testing in Europe were found to be allergic to the oxidized form of linalool.[18]
See also
References
- "Linalool". Dictionary.com Unabridged. Random House. Retrieved 2016-01-22.
- "Linalool". PubChem, US National Library of Medicine. 12 February 2017. Retrieved 14 February 2017.
- "linalool". Merriam-Webster Dictionary.: "International Scientific Vocabulary, from Mexican Spanish lináloe"
- Woronuk G, Demissie Z, Rheault M, Mahmoud S (January 2011). "Biosynthesis and therapeutic properties of Lavandula essential oil constituents". Planta Medica. 77 (1): 7–15. doi:10.1055/s-0030-1250136. PMID 20665367.
- Dewick PM (2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). John Wiley & Sons. ISBN 978-0-470-74168-9.
- Cseke L, Dudareva N, Pichersky E (November 1998). "Structure and evolution of linalool synthase". Molecular Biology and Evolution. 15 (11): 1491–8. doi:10.1093/oxfordjournals.molbev.a025876. PMID 12572612.
- Iijima Y, Davidovich-Rikanati R, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E (November 2004). "The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil". Plant Physiology. 136 (3): 3724–36. doi:10.1104/pp.104.051318. PMC 527170. PMID 15516500.
- "Widely Used Fragrance Ingredients In Shampoos And Conditioners Are Frequent Causes Of Eczema". MedicalNewsToday. 28 March 2009. Archived from the original on 9 January 2010.
- Yang Z, Bengtsson M, Witzgall P (March 2004). "Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella". Journal of Chemical Ecology. 30 (3): 619–29. doi:10.1023/b:joec.0000018633.94002.af. PMID 15139312.
- "What to look for when you're buying mosquito repellent". South China Morning Post. September 6, 2015. Retrieved December 30, 2015.
- "EPA Linalool Summary Document Registration Review: Initial Docket" (PDF). U.S. Environmental Protection Agency. April 2007.
- Kasper S, Gastpar M, Müller WE, Volz HP, Möller HJ, Dienel A, Schläfke S (September 2010). "Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of 'subsyndromal' anxiety disorder: a randomized, double-blind, placebo controlled trial". International Clinical Psychopharmacology. 25 (5): 277–87. doi:10.1097/YIC.0b013e32833b3242. PMID 20512042. S2CID 46290020.
- Ahmed A, Choudhary MI, Farooq A, Demirci B, Demirci F, Can Başer KH (2000). "Essential oil constituents of the spice Cinnamomum tamala (Ham.) Nees & Eberm". Flavour and Fragrance Journal. 15 (6): 388–390. doi:10.1002/1099-1026(200011/12)15:6<388::AID-FFJ928>3.0.CO;2-F.
- Ibrahim EA, Wang M, Radwan MM, Wanas AS, Majumdar CG, Avula B, et al. (March 2019). "Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application". Planta Medica. 85 (5): 431–438. doi:10.1055/a-0828-8387. PMID 30646402.
- Klimankova E, Holadová K, Hajšlová J, Čajka T, Poustka J, Koudela M (2008). "Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions". Food Chemistry. 107 (1): 464–472. doi:10.1016/j.foodchem.2007.07.062.
- Vila R, Mundina M, Tomi F, Furlán R, Zacchino S, Casanova J, Cañigueral S (February 2002). "Composition and antifungal activity of the essential oil of Solidago chilensis". Planta Medica. 68 (2): 164–7. doi:10.1055/s-2002-20253. PMID 11859470.
- "Linalool". Toxnet, National Library of Medicine, US National Institutes of Health. 14 January 2016. Archived from the original on 28 February 2019. Retrieved 21 May 2019.
- Ung CY, White JM, White IR, Banerjee P, McFadden JP (March 2018). "Patch testing with the European baseline series fragrance markers: a 2016 update". The British Journal of Dermatology. 178 (3): 776–780. doi:10.1111/bjd.15949. PMID 28960261. S2CID 4434234.
External links
- Comprehensive data sheet
- Record in the Household Products Database of NLM
