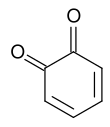
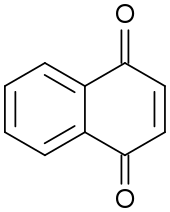
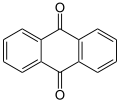
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure".[1] The class includes some heterocyclic compounds.
The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.
Properties
Quinones are oxidized derivatives of aromatic compounds and are often readily made from reactive aromatic compounds with electron-donating substituents such as phenols and catechols, which increase the nucleophilicity of the ring and contributes to the large redox potential needed to break aromaticity. (Quinones are conjugated but not aromatic). Quinones are electrophilic Michael acceptors stabilised by conjugation. Depending on the quinone and the site of reduction, reduction can either rearomatise the compound or break the conjugation. Conjugate addition nearly always breaks the conjugation.
The term quinone is also used more generally for a large class of compounds formally derived from aromatic quinones through replacement of some hydrogen atoms by other atoms or radicals.
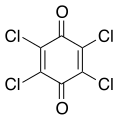 Chloranil, a reagent in organic chemistry
Chloranil, a reagent in organic chemistry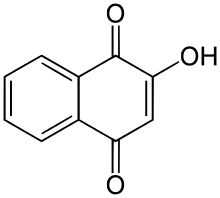 Lawsone, a dye present in the leaves of the henna plant
Lawsone, a dye present in the leaves of the henna plant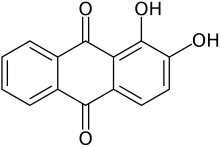 Alizarin, a common red dye
Alizarin, a common red dye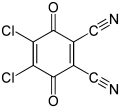 DDQ, a reagent in organic chemistry
DDQ, a reagent in organic chemistry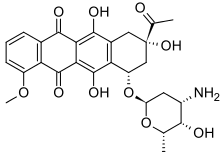 Daunorubicin, an anticancer drug
Daunorubicin, an anticancer drug
Occurrence and uses
Production of hydrogen peroxide
A large scale industrial application of quinones is for the production of hydrogen peroxide. 2-Alkylanthraquinones are hydrogenated to the corresponding hydroquinones (quinizarins), which then transfer H
2 to oxygen:
- dihydroanthraquinone + O
2 → anthraquinone + H
2O
2
in this way, several billion kilograms of H
2O
2 are produced annually.[2]
Biochemistry
Derivatives of quinones are common in biologically active molecules. They are conjectured to occur in all respiring organisms.[3] Some serve as electron acceptors in electron transport chains such as those in photosynthesis (plastoquinone, phylloquinone), and aerobic respiration (ubiquinone). Phylloquinone is also known as Vitamin K1 as it is used by animals to carboxylate certain proteins, which are involved in blood coagulation, bone formation, and other processes. Conversely, the toxicity of paracetamol is due to its metabolism to a quinone imine, which then reacts with liver proteins to cause liver failure.
In the spray of bombardier beetles, hydroquinone reacts with hydrogen peroxide to produce a fiery blast of steam, a deterrent in the animal world.
The auto-oxidation of the neurotransmitter dopamine and its precursor L-Dopa generates the comparatively stable dopamine quinone which inhibits the functioning of dopamine transporter (DAT) and the TH enzyme leading to low mitochondrial ATP production.[4]
Medicinal
Several quinones are of pharmacological interest. They form a major class of cytotoxins, used in the fight against cancers. One example is daunorubicin, which is antileukemic.[3] Some of them show anti-tumoral activity. They embody some claims in herbal medicine. These applications include purgative (sennosides), antimicrobial and antiparasitic (rhein and saprorthoquinone, atovaquone), anti-tumor (emodin and juglone), inhibition of PGE2 biosynthesis (arnebinone and arnebifuranone) and anti-cardiovascular disease (tanshinone).[5]
Dyes
Many natural and artificial coloring substances (dyes and pigments) are quinone derivatives. They are second only to azo dyes in importance as dyestuffs, with particular emphasis on blue colors. Alizarin (1,2-dihydroxy-9,10-anthraquinone), extracted from the madder plant, was the first natural dye to be synthesized from coal tar.
Reagents in organic chemistry
Benzoquinone is used in organic chemistry as an oxidizing agent. Strongly oxidizing quinones include chloranil and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (also known as DDQ).[6]
Battery charge carrier
9,10-Anthraquinone-2,7-disulphonic acid (AQDS) a quinone similar to one found naturally in rhubarb has been used as a charge carrier in metal-free flow batteries.[7]
Nomenclature
Quinones are commonly named with a prefix that indicates the parent aromatic hydrocarbon ("benzo-" for benzene, "naphtho-" for naphthalene, "anthra-" for anthracene, etc.) and the "-quinone" suffix. Infix multipliers "-di-", "-tri-", "-tetra-" (etc.) are used when there are 4, 6, 8 (etc.) carbonyls. The position of the carbonyl groups can be indicated before the prefix (as in "1,4,5,8-naphthodiquinone") or after it ("anthra-1,4-quinone").
See also
- Anthraquinone
- Benzoquinone
- Naphthoquinone
- Plastoquinone
- Pyrroloquinoline quinone
- Quinone methide
- Quinonediimine – nitrogen analogue
- Malbranchea cinnamomea
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Quinones". doi:10.1351/goldbook.Q05015
- Gustaaf Goor, Jürgen Glenneberg, Sylvia Jacobi "Hydrogen Peroxide" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a13_443.pub2.
- O'Brien, P.J. (1991). "Molecular mechanisms of quinone cytotoxicity". Chemico-Biological Interactions. 80 (1): 1–41. doi:10.1016/0009-2797(91)90029-7. PMID 1913977.
- Dorszewska, Jolanta; Prendecki, Michal; Kozubski, Margarita Lianeri and Wojciech (2014-01-31). "Molecular Effects of L-dopa Therapy in Parkinson's Disease". Current Genomics. 15 (1): 11–17. doi:10.2174/1389202914666131210213042. PMC 3958954. PMID 24653659.
- Liu H., "Extraction and Isolation of Compounds from Herbal Medicines" in 'Traditional Herbal Medicine Research Methods', ed by Willow JH Liu 2011 John Wiley and Sons, Inc.
- March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- Huskinson, Brian; Marshak, Michael P.; Suh, Changwon; Er, Süleyman; Gerhardt, Michael R.; Galvin, Cooper J.; Chen, Xudong; Aspuru-Guzik, Alán; Gordon, Roy G.; Aziz, Michael J. (9 January 2014). "A metal-free organic-inorganic aqueous flow battery" (PDF). Nature. 505 (7482): 195–198. Bibcode:2014Natur.505..195H. doi:10.1038/nature12909. PMID 24402280.
External links
- Quinones at the US National Library of Medicine Medical Subject Headings (MeSH)