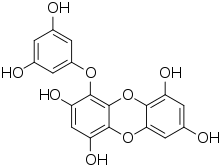
Phlorotannin
Phlorotannins are a type of tannins found in brown algae such as kelps and rockweeds[1] or sargassacean species,[2] and in a lower amount also in some red algae.[3] Contrary to hydrolysable or condensed tannins, these compounds are oligomers of phloroglucinol[4] (polyphloroglucinols).[5] As they are called tannins, they have the ability to precipitate proteins. It has been noticed that some phlorotannins have the ability to oxidize and form covalent bonds with some proteins. In contrast, under similar experimental conditions three types of terrestrial tannins (procyanidins, profisetinidins, and gallotannins) apparently did not form covalent complexes with proteins.[6]
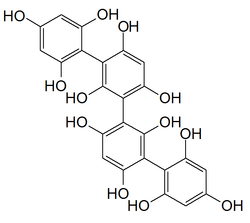

These phenolic compounds are integral structural components of cell walls in brown algae, but they also seem to play many other secondary ecological roles such as protection from UV radiation and defense against grazing.
Biosynthesis and localization
Most of the phlorotannins' biosynthesis is still unknown, but it appears they are formed from phloroglucinols via the acetate-malonate pathway.[7]
They are found within the cell in small vesicles called physodes, where the soluble, polar fraction is sequestrated,[8] and as part of the cell wall, where they are insoluble and act as a structural component.[9][10] Their concentration is known to be highly variable among different taxa as well as among geographical area, since they respond plastically to a variety of environmental factors.[11] Brown algaes also exsude phlorotannins in surrounding seawater.[5][12]
It has been proposed that phlorotannins are first sequestered in physodes under their polar, reactive form before being oxidized and complexed to the alginic acid of brown algal cell wall by a peroxidase.[13] To this date (2012), not much is known about phlorotannins synthesis.[7] The formation of physodes, vesicles containing phenolic compounds, have been investigated for many years. These cytoplasmic constituents were thought to be synthesized in the chloroplast or its membrane, but more recent studies suggest that the formation may be related to the endoplasmic reticulum and Golgi bodies.[14]
The allocation of phlorotannins among tissues varies along with the species.[15]
The localization of phlorotannins can be investigated by light microscopy after vanillin–HCl staining giving an orange color.[16] The ultrastructural localization of physodes can be examined through transmission electron microscopy in samples primarily fixed in 2.5% glutaraldehyde and with postfixation with 1% osmium tetroxide. For staining, uranyl acetate and lead citrate can be used.
Extraction and assays
In many studies where individual phlorotannins are isolated, extracted phlorotannins are acetylated with acetic anhydride-pyridine to protect them from oxidation. Both lowering the temperature and the addition of ascorbic acid seem to prevent oxidation.
Usual assays to quantify phlorotannins in samples are the Folin-Denis and Prussian blue assays. A more specific assay makes use of 2,4-dimethoxybenzaldehyde (DMBA), a product that reacts specifically with 1,3-and 1,3,5-substituted phenols (e.g., phlorotannins) to form a colored product.[17]
Structural diversity
The nomenclature system for the marine phlorotannins was originally introduced by Glombitza.[18]
Phlorotannins are classified following the arrangement of the phloroglucinol monomeres. More than 150 compounds are known, ranging from 126 Da to 650 kDa in molecular weight.[8][19] Most of them are found between 10 and 100kDa.[20]
They are distributed in six main subgroups: fucols, phlorethols, fucophloretols, fuhalols and eckols, which are only found in the Alariaceae.[7][21]
According to linkage type, phlorotannins can be classified into four subclasses, i.e., phlorotannins with an ether linkage (fuhalols and phlorethols, fuhalols are constructed of phloroglucinol units that are connected with para- and ortho-arranged ether bridges containing one additional OH-group in every third ring), with a phenyl linkage (fucols), with an ether and a phenyl linkage (fucophlorethols) and with a dibenzodioxin linkage in eckols and carmalols (derivatives of phlorethols containing a dibenzodioxin moiety), most of which have halogenated representatives in brown algae.[22]
Examples of phlorotannins are fucodiphlorethol G from the seaweed Ecklonia cava,[23] eckol from Ecklonia species[24] or phlorofucofuroeckol-B from Eisenia arborea.[25]
The structural diversity of higher molecular weight molecules can be screened through the use of the 'EDIT' Carbon-13 NMR technique.[26]
Roles
The functions of phlorotannins are still an actual research subject (2012). They show primary and secondary roles,[13] at both cellular and organismic scale.[27]
Primary roles
Structural
The structural role of phlorotannins in brown algal cell wall is a primary role of these polyphenolic compounds.[7][13] This primary role may however not be the main role of the phlorotannins, since studies show they are more abundant in cytoplasm or in the exuded form than in cell wall.[28]
Reproductive
Cytoplasmic as well as exuded phlorotannins seem to play a role in algal reproduction, by contributing to the formation of the zygote's cell wall[10] and perhaps avoiding multiple fertilization by inhibiting spermatozoid movement.[7]
Secondary roles
According to the Carbon Nutrient Balance Model, phlorotannins, which are predominantly carbon molecules free of nitrogen, are produced in higher yields in light environment. Light has greater importance than nitrogen availability.[29]
Studies shown that phlorotannins seem to act as a protection for brown algaes in a number of ways. Here are some examples.
Antiherbivory defense
Phlorotannin production strategy may be constitutive or inducible.[30] As studies demonstrated that herbivory can induce phlorotannin production, it has been suggested that they may have a role in algae defense.[11] However, results form other studies suggest that the deterrent role of phlorotannins on herbivory is highly dependent on both algae and herbivore species.[31] In Fucus vesiculosus, it is galactolipids, rather than phlorotannins, that act as herbivore deterrents against the sea urchin Arbacia punctulata.[32]
UV and heavy metals screening
Phlorotannins are mostly located at the periphery of the cells, as components of the cell wall. They also contribute to absorption of UV-B light (between 280 and 320 nm)[33] and show absorbance maxima at 200 and 265 nm,[8] corresponding to UV-C wavelengths. Studies also demonstrated that sunlight intensity is related to phlorotannins production in Ascophyllum nodosum and Fucus vesiculosus natural populations.[34] For these reasons, it has been suggested that phlorotannins act as photoprotective substances.[35] Further studies with Lessonia nigrescens[35] and Macrocystis integrifolia[36] demonstrated that both UV-A and UV-B radiation can induce soluble phlorotannins and that there is a correlation between induction of phlorotannins and reduction in the inhibition of photosynthesis and DNA damage, two major effetcts of UV radiation on vegetal tissues. The fact that phlorotannins are exudated in surrounding water enables them to reduce incident UV exposure on kelp meiospores, phytoplankton and other kelp forests inhabitants, where brown algal biomass is high and water motion is low.[36]
They may also be involved in metal sequestration such as divalent metal ions Sr2+, Mg2+, Ca2+, Be2+, Mn2+, Cd2+, Co2+, Zn2+, Ni2+, Pb2+ and Cu2+.[37] If the chelating properties of phlorotannins have been demonstrated in vitro, in situ studies suggest that this characteristic may be species-specific.[38][39]
Therapeutic properties
It has demonstrated that phlorotannins can have anti-diabetic, anti-cancer, anti-oxidation, antibacterial, radioprotective and anti-HIV properties.[41][42] However, in vivo studies on the effects of these compounds are lacking, most of the research having so far been done in vitro.[41] Regarding anti-allergic property, there is in vivo study on the effect of these compounds.[43]
References
- Van Alstyne, Kathryn L.; McCarthy, James J.; Hustead, Cynthia L.; Kearns, Laura J. (1999). "Phlorotannin Allocation Among Tissues of Northeastern Pacific Kelps and Rockweeds" (PDF). Journal of Phycology. 35 (3): 483. doi:10.1046/j.1529-8817.1999.3530483.x. Archived from the original (PDF) on 2010-06-19.
- Kamiya, Mitsunobu; Nishio, Takeshi; Yokoyama, Asami; Yatsuya, Kousuke; Nishigaki, Tomokazu; Yoshikawa, Shinya; Ohki, Kaori (2010). "Seasonal variation of phlorotannin in sargassacean species from the coast of the Sea of Japan". Phycological Research. 58: 53. doi:10.1111/j.1440-1835.2009.00558.x.
- http://eurekamag.com/research/011/190/presence-lectins-tannins-protease-inhibitors-venezuelan-marine-algae.php
- Shibata, Toshiyuki; Kawaguchi, Shigeo; Hama, Yoichiro; Inagaki, Masanori; Yamaguchi, Kuniko; Nakamura, Takashi (2004). "Local and chemical distribution of phlorotannins in brown algae". Journal of Applied Phycology. 16 (4): 291. doi:10.1023/B:JAPH.0000047781.24993.0a.
- Ragan, Mark A.; Jensen, Arne (1978). "Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. And Fucus vesiculosus (L.)". Journal of Experimental Marine Biology and Ecology. 34 (3): 245. doi:10.1016/S0022-0981(78)80006-9.
- Stern, J. Lewis; Hagerman, Ann E.; Steinberg, Peter D.; Mason, Pamela K. (1996). "Phlorotannin-protein interactions". Journal of Chemical Ecology. 22 (10): 1877–99. doi:10.1007/BF02028510. PMID 24227114.
- Riitta Koivikko, 2008, Brown algal phlorotannins: Improving and applying chemical methods Archived 2016-03-03 at the Wayback Machine, Ph. D. Thesis, University of Turku, Turku, Finland.
- Ragan, Mark A.; Glombitza, K.-W. (1986). "Phlorotannins, brown algal polyphenols". Prog. Phycol. Res. 4: 129–241.
- Schoenwaelder, M. E. A. (2002). "The occurrence and cellular significance of physodes in brown algae". Phycologia. 41: 125–139. doi:10.2216/i0031-8884-41-2-125.1.
- Schoenwaelder, M. E. A.; Clayton, M. N. (1998). "Secretion of phenolic substances into the zygote wall and cell plate in embryos of Hormosira and Acrocarpis (Fucales, Phaeophyceae)". Journal of Phycology. 34: 969–980. doi:10.1046/j.1529-8817.1998.340969.x.
- Jormalainen, V.; Honkanen, T.; Koivikko, R.; Eränen, J. (2003). "Induction of phlorotannin production in a brown alga: defense or resource dynamics?" (PDF). Oikos. 103: 640–650. doi:10.1034/j.1600-0706.2003.12635.x.
- Jennings, J.S.; Steinberg, P.D. (1994). "In situ exudation of phlorotannins by the sublittoral kelp Ecklonia radiata". Mar. Biol. 121: 349–354. doi:10.1007/bf00346744.
- Arnold, T. M.; Targett, N. M. (2003). "To grow and defend: lack of tradeoffs for brown algal phlorotannins". Oikos. 100 (2): 406–408. doi:10.1034/j.1600-0706.2003.11680.x.
- Schoenwaelder, M.E.A.; Clayton, M.N. (2000). "Physode formation in embryos of Phyllospora comosa and Hormosira banksii (Phaeophyceae)". Phycologia. 39: 1–9. doi:10.2216/i0031-8884-39-1-1.1.
- Van Alstyne, Kathryn L. (1999). "PHLOROTANNIN ALLOCATION AMONG TISSUES OF NORTHEASTERN PACIFIC KELPS AND ROCKWEEDS". Journal of Phycology. 35: 483–492. doi:10.1046/j.1529-8817.1999.3530483.x.
- Pellegrini, L (1980). "Cytological studies on physodes in the vegetative cells of Cystoseira stricter Sauvagea (Phaeophyta, Fucales)". J. Cell Sci. 41: 209–231.
- Stern, J. Lewis (1996). "A new assay for quantifying brown algal phlorotannins and comparisons to previous methods". Journal of Chemical Ecology. 22: 1273–1293. doi:10.1007/BF02266965. PMID 24226084.
- Glombitza, K. W. In Marine Natural Product Chemistry; Faulkner, D. J.; Fenical, W. H., Eds.; Plenum Press: New York, 1977; p 191.
- Hay, M E; Fenical, W (1988). "Marine Plant-Herbivore Interactions: The Ecology of Chemical Defense". Annual Review of Ecology and Systematics. 19: 111. doi:10.1146/annurev.es.19.110188.000551.
- Boettcher, A.A.; Targett, N.M. (1993). "Role of polyphenolic molecular-size in reduction of assimilation efficiency in Xiphister mucosus". Ecology. 74: 891–903. doi:10.2307/1940814.
- Targett, N.M.; Arnold, T. M. (1998). "Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans". Journal of Phycology. 34: 195–205. doi:10.1046/j.1529-8817.1998.340195.x.
- La Barre, Stéphane; Potin, Philippe; Leblanc, Catherine; Delage, Ludovic (2010). "The Halogenated Metabolism of Brown Algae (Phaeophyta), Its Biological Importance and Its Environmental Significance". Marine Drugs. 8 (4): 988–1010. doi:10.3390/md8040988. PMC 2866472. PMID 20479964.
- Young Min Ham, Jong Seok Baik, Jin Won Hyun and Nam Ho Lee, Bull. 2007. Isolation of a new phlorotannin, fucodiphlorethol G, from a brown alga Ecklonia cava Archived 2012-04-25 at the Wayback Machine. Korean Chem. Soc. 28(9): 1595.
- Moon, Changjong; Kim, Sung-Ho; Kim, Jong-Choon; Won Hyun, Jin; Ho Lee, Nam; Woo Park, Jae; Shin, Taekyun (2008). "Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice". Phytotherapy Research. 22 (2): 238–242. doi:10.1002/ptr.2298. PMID 17886227.
- Sugiura, Yoshimasa; Matsuda, Kohji; Yamada, Yasuhiro; Nishikawa, Masashi; Shoiya, Kazufumi; Katsuzaki, Hirotaka; Imai, Kunio; Amano, Hideomi (2006). "Isolation of a New Anti-Allergic Phlorotannin, Phlorofucofuroeckol-B, from an Edible Brown Alga, Eisenia arborea". Biosci. Biotechnol. Biochem. 70 (11): 2807–11. doi:10.1271/bbb.60417. PMID 17090915.
- McInnes, A. G. (1984). "High-molecular-weight phloroglucinol-based tannins from brown algae: Structural variants". Hydrobiologia. 116–117: 597–602. doi:10.1007/BF00027755.
- Schoenwaelder, Monica E. A. (2002). "The occurrence and cellular significance of physodes in brown algae". Phycologia. 41 (2): 125–139. doi:10.2216/i0031-8884-41-2-125.1.
- Koivikko, Riitta; Loponen, Jyrki; Honkanen, Tuija; Jormalainen, Veijo (2005). "Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions". Journal of Chemical Ecology. 31 (1): 195–212. CiteSeerX 10.1.1.320.5895. doi:10.1007/s10886-005-0984-2. PMID 15839490.
- Pavia, Henrik; Toth, Gunilla B. (2000). "Influence of light and nitrogen on the phlorotannin content of the brown seaweeds Ascophyllum nodosum and Fucus vesiculosus". Hydrobiologia. 440 (1–3): 299–305.
- Hammerstrom, Kamille; Dethier, Megan N.; Duggins, David O. (1998). "Rapid phlorotannin induction and relaxation in five Washington kelps" (PDF). Mar. Ecol. Prog. Ser. 165: 293–305. doi:10.3354/meps165293.
- Amsler, C.D.; Fairhead, V.A. (2006). "Defensive and sensory chemical ecology of brown algae". Adv. Bot. Res. 43: 1–91.
- Deal, Michael S. (2003). "Galactolipids rather than phlorotannins as herbivore deterrents in the brown seaweed Fucus vesiculosus". Oecologia. 136: 107–114. doi:10.1007/s00442-003-1242-3. PMID 12684854.
- Pavia, H.; Cervin, G.; Lindgren, A.; Aberg, Per (1997). "Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum". Marine Ecology Progress Series. 157: 139–146. doi:10.3354/meps157139.
- Pavia, Henrik; Toth, Gunilla B. (2000). "Influence of light and nitrogen on the phlorotannin content of the brown seaweeds Ascophyllum nodosum and Fucus vesiculosus". Hydrobiologia. 440: 299–305. doi:10.1023/A:1004152001370.
- Gómez, Ivan; Huovinen, Pirjo (2010). "Induction of Phlorotannins During UV Exposure Mitigates Inhibition of Photosynthesis and DNA Damage in the Kelp Lessonia nigrescens". Photochemistry and Photobiology. 86 (5): 1056–63. doi:10.1111/j.1751-1097.2010.00786.x. PMID 20670358.
- Swanson, Andrew K; Druehl, Louis D (2002). "Induction, exudation and the UV protective role of kelp phlorotannins". Aquatic Botany. 73 (3): 241. doi:10.1016/S0304-3770(02)00035-9.
- Ragan, Mark A; Smidsrød, Olav; Larsen, Bjørn (1979). "Chelation of divalent metal ions by brown algal polyphenols". Marine Chemistry. 7 (3): 265. doi:10.1016/0304-4203(79)90043-4.
- Huovinen, Pirjo; Leal, Pablo; Gómez, Iván (2010). "Interacting effects of copper, nitrogen and ultraviolet radiation on the physiology of three south Pacific kelps". Marine and Freshwater Research. 61 (3): 330. doi:10.1071/MF09054.
- Toth, G; Pavia, H (2000). "Lack of phlorotannin induction in the brown seaweed Ascophyllum nodosum in response to increased copper concentrations". Marine Ecology Progress Series. 192: 119–126. doi:10.3354/meps192119. INIST:1367809.
- Nagayama, Koki; Shibata, Toshiyuki; Fujimoto, Ken; Honjo, Tuneo; Nakamura, Takashi (2003). "Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae". Aquaculture. 218 (1–4): 601. doi:10.1016/S0044-8486(02)00255-7.
- Gupta, Shilpi; Abu-Ghannam, Nissreen (2011). "Bioactive potential and possible health effects of edible brown seaweeds". Trends in Food Science & Technology. 22 (6): 315. CiteSeerX 10.1.1.465.6140. doi:10.1016/j.tifs.2011.03.011.
- Li, Yong-Xin; Wijesekara, Isuru; Li, Yong; Kim, Se-Kwon (2011). "Phlorotannins as bioactive agents from brown algae". Process Biochemistry. 46 (12): 2219. doi:10.1016/j.procbio.2011.09.015.
- Sugiura, Yoshimasa; Usui, Masakatsu; Katsuzaki, Hirotaka; Imai, Kunio; Kakinuma, Makoto; Amano, Hideomi; Miyata, Masaaki (2018). "Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling". Marine Drugs. 16 (8): 267. doi:10.3390/md16080267. PMC 6117712. PMID 30072652.
External links
- Riitta Koivikko. 2008. Brown algal phlorotannins: Improving and applying chemical methods, Ph. D. Thesis, University of Turku, Turku, Finland.
- Gupta, Shilpi; Abu-Ghannam, Nissreen (2011). "Bioactive potential and possible health effects of edible brown seaweeds". Trends in Food Science & Technology. 22 (6): 315. CiteSeerX 10.1.1.465.6140. doi:10.1016/j.tifs.2011.03.011.