Chlorophyll c
Chlorophyll c is a form of chlorophyll found in certain marine algae, including the photosynthetic Chromista (e.g. diatoms, brown algae) and dinoflagellates.[1][2][3]
It has a blue-greenish color and is an accessory pigment, particularly significant in its absorption of light in the 447-452 nm wavelength region, [3] Like chlorophyll a and chlorophyll b, it helps the organism gather light and passes a quanta of excitation energy through the light harvesting antennae to the photosynthetic reaction centre. Chlorophyll c is unusual because it has a porphyrin ring structure and does not have an isoprenoid tail or a reduced ring D, features typical of the other chlorophylls commonly found in algae and plants.[2]
Chlorophyll c can be further divided into chlorophyll c1, chlorophyll c2[3] and chlorophyll c3,[4] plus at least 8 other more-recently-found subtypes.[5]
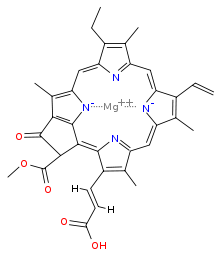
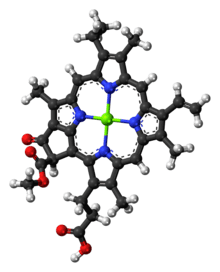
Chlorophyll c1
 | |
 | |
| Names | |
|---|---|
| IUPAC name
[(2E)-3-[14-Ethyl-21-(methoxycarbonyl)-4,8,13,18-tetramethyl-20-oxo-9-vinyl-3,4-didehydro-3-phorbinyl-κ2N23,N25]acrylato(2-)]magnesium | |
| Identifiers | |
3D model (JSmol) |
|
| 5801077, 6996880 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C35H30MgN4O5 | |
| Molar mass | 610.953 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorophyll c1 is a common form of chlorophyll c. It differs from chlorophyll c2 in its C8 group, having an ethyl group instead of vinyl group (C-C single bond instead of C=C double bond). Its absorption maxima are around 444, 577, 626 nm and 447, 579, 629 nm in diethyl ether and acetone respectively.[6]
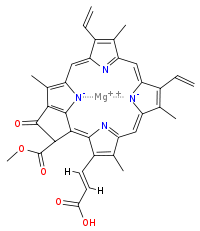
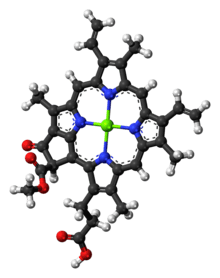
Chlorophyll c2
 | |
 | |
| Names | |
|---|---|
| IUPAC name
[(2E)-3-[21-(Methoxycarbonyl)-4,8,13,18-tetramethyl-20-oxo-9,14-divinyl-3,4-didehydro-3-phorbinyl-κ2N23,N25]acrylato(2-)]magnesium | |
| Identifiers | |
3D model (JSmol) |
|
| 5801049 6996841 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C35H28MgN4O5 | |
| Molar mass | 608.937 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorophyll c2 is the most common form of chlorophyll c.[7] Its absorption maxima are around 447, 580, 627 nm and 450, 581, 629 nm in diethyl ether and acetone respectively.[6]
Chlorophyll c3
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C36H28MgN4O7 | |
| Molar mass | 652.946 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorophyll c3 is a form of chlorophyll c found in microalga Emiliania huxleyi, identified in 1989.[4] Its absorption maxima are around 452, 585, 625 nm and 452, 585, 627 nm in diethyl ether and acetone respectively.[6]
References
- Speer BR. "Photosynthetic Pigments". Retrieved 2 August 2014.
- Blankenship RE (February 2002). Molecular Mechanisms of Photosynthesis. Wiley-Blackwell.
- Dougherty RC, Strain HH, Svec WA, Uphaus RA, Katz JJ (May 1970). "The structure, properties, and distribution of chlorophyll c". Journal of the American Chemical Society. 92 (9): 2826–33. doi:10.1021/ja00712a037. PMID 5439971.
- Fookes CJ, Jeffrey SW (1989). "The structure of chlorophyll c3, a novel marine photosynthetic pigment". J. Chem. Soc., Chem. Commun. (23): 1827–1828. doi:10.1039/C39890001827.
- Zapata M, Garrido JL, Jeffrey SW (2006). "Chlorophyll c Pigments: Current Status". Chlorophylls and Bacteriochlorophylls: Advances in Photosynthesis and Respiration. Advances in Photosynthesis and Respiration. 25: 39–53. doi:10.1007/1-4020-4516-6_3. ISBN 978-1-4020-4515-8.
- Fawley MW (October 1989). "A new form of chlorophyll C involved in light-harvesting". Plant Physiology. 91 (2): 727–32. doi:10.1104/pp.91.2.727. PMC 1062062. PMID 16667093.
- Jeffrey SW (September 1976). "The Occurrence of Chlorophyll c1 and c2 in Algae". Journal of Phycology. 12 (3): 349–354. doi:10.1111/j.1529-8817.1976.tb02855.x.