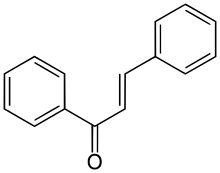
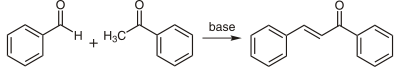Chalcone
Chalcone is an aromatic ketone and an enone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones or chalconoids. Alternative names for chalcone include benzylideneacetophenone, phenyl styryl ketone, benzalacetophenone, β-phenylacrylophenone, γ-oxo-α,γ-diphenyl-α-propylene, and α-phenyl-β-benzoylethylene.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Chalcone[2] | |
| Systematic IUPAC name
(2E)-1,3-Diphenylprop-2-en-1-one | |
| Other names
Chalkone Benzylideneacetophenone Phenyl styryl ketone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.119 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12O | |
| Molar mass | 208.260 g·mol−1 |
| Density | 1.071 g/cm3 |
| Melting point | 55 to 57 °C (131 to 135 °F; 328 to 330 K) |
| Boiling point | 345 to 348 °C (653 to 658 °F; 618 to 621 K) |
| -125.7·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemical properties
Chalcones have two absorption maximums at 280 nm and 340 nm.[3]
Chemical reactions
Synthesis
Chalcones can be prepared by an aldol condensation between benzaldehyde and acetophenone in the presence of sodium hydroxide as a catalyst.[4][5]
This reaction can be carried out without any solvent as a solid-state reaction.[6] The reaction between substituted benzaldehydes and acetophenones can be used as an example of green chemistry in undergraduate education.[7] In a study investigating green syntheses, chalcones were synthesized from the same starting materials in high-temperature water (200 to 350 °C).[8]
Substituted chalcones were also synthesised by piperidine-mediated condensation to avoid side reactions such as multiple condensations, polymerizations, and rearrangements.[9]
Other reactions
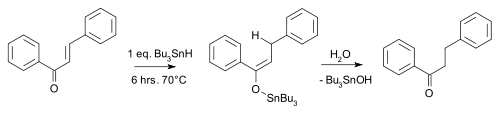
An example is the conjugate reduction of the enone by tributyltin hydride:[10]
3,5-Disubstituted 1H-pyrazoles can be produced from a suitably substituted chalcone by reaction with hydrazine hydrate in the presence of elemental sulfur[11] or sodium persulfate,[12] or by using a hydrazone in which case an azine is produced as a by-product. The specific case for formation of 3,5-diphenyl-1H-pyrazole from chalcone itself can be represented as:[13]
Potential pharmacology
Chalcones and their derivatives demonstrate a wide range of biological activities including anti-inflammation.[14] Some 2′-amino chalcones are have been studied as potential antitumor agents.[15][16] The therapeutic (anti-cancer, anti-bacterial, anti-fungal, anti-viral, anti-ameobic, anti-malarial, anti-tubercular, nematicidal, anti-oxidant, inhibitors against various therapeutic targets, etc.), catalytic, chemosensing, and photosensitizing potentials of various Metal (Iron, Ruthenium, Platinum, Copper, Zinc, Cobalt, Manganese, Nickel, Osmium, Chromium, Tellurium, Boron, Tungsten, and Silicon)-Chalcone complexes have also been reported. [17]
Biological Target Inhibition Perspectives
Several natural and (semi) synthetic chalcones have shown anti-cancer activity due to their inhibitory potential against various targets namely ATP-binding cassette super-family G member 2 (ABCG2), P-glycoprotein (P-gp), Breast Cancer Resistance Protein (BCRP), 5α-reductase, aromatase, 17-β-hydroxysteroid dehydrogenase, histone deacetylase (HDAC)/Sirtuin-1, proteasome, vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor-2 (VEGFR-2) kinase, matrix metalloproteinases (MMP)-2/9, Janus kinases (JAK)/Signal transducer and activator of transcription proteins (STAT) signaling pathways, Cell Division Cycle-25 (CDC25B), tubulin, cathepsin-K, topoisomerase-II, Wingless-related integration site (Wnt), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κβ), v-raf murine sarcoma viral oncogene homolog B1 (B-Raf), mammalian target of rapamycin (mTOR), etc. [18] Chalcone molecules deserve the credit of being potential anti-diabetic candidates that act by modulating the therapeutic targets Peroxisome proliferator-activated receptor gamma (PPAR-Γ), Dipeptidyl peptidase-4 (DPP-4), α-glucosidase, Protein-tyrosine Phosphatase 1B (PTP1B), aldose reductase, and tissue sensitivity. [19] Chalcones have been identified as the potential anti-infective candidates that inhibit various parasitic, malarial, bacterial, viral, and fungal targets like cruzain-1/2, trypanopain-Tb, trans-sialidase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), fumarate reductase, falcipain-1/2, β-hematin, topoisomerase-II, plasmepsin-II, lactate dehydrogenase, protein kinases (Pfmrk and PfPK5), sorbitol-induced hemolysis, recombinant dengue virus Type 1 (DEN-1 NS3), influenza A virus (H1N1), human immunodeficiency virus (HIV-Integrase/Protease), protein tyrosine phosphatase A/B (Ptp-A/B), filamentous temperature-sensitive mutant Z (FtsZ), fatty acid syntheses (FAS-II), lactate/isocitrate dehydrogenase, NorA efflux pump, deoxyribonucleic acid (DNA) gyrase, fatty acid synthase, chitin synthase, β-(1,3)-glucan synthase, etc. [20] Chalcones are the promising candidates in inhibiting various cardiovascular, hematological and anti-obesity targets like angiotensin-converting enzyme (ACE), cholesteryl ester transfer protein (CETP), diacylglycerol acyltransferase (DGAT), acyl-coenzyme A: cholesterol acyltransferase (ACAT), pancreatic lipase (PL), lipoprotein lipase (LPL), calcium (Ca2+)/potassium (K+) channel, thromboxane (TXA2 and TXB2), etc. [21] Chalcone derivatives have demonstrated admirable anti-inflammatory activity due to their inhibitory potential against various therapeutic targets like cyclooxygenase (COX), lipooxygenase (LOX), interleukins (IL), prostaglandins (PGs), nitric oxide synthase (NOS), leukotriene D4 (LTD4), nuclear factor-κB (NFκB), intracellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), monocyte chemoattractant protein-1 (MCP-1), TLR4/MD-2, etc. [22]
See also
References
- Merck Index, 11th Edition, 2028
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 722. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Song, Dong-mee; Jung, Kyoung-Hoon; Moon, Ji-hye; Shin, Dong-Myung (2003). "Photochemistry of chalcone and the application of chalcone-derivatives in photo-alignment layer of liquid crystal display". Optical Materials. 21 (1–3): 667–71. Bibcode:2003OptMa..21..667S. doi:10.1016/S0925-3467(02)00220-3.
- Dumitru, Sîrbu; Ion, Marin (2011). "SYNTHESIS AND IR, NMR CARACTERISATION OF NEW P-(N,N-DIPHENYLAMINO) CHALCONES". Cite journal requires
|journal=(help) - Gómez-Rivera, Abraham; Aguilar-Mariscal, Hidemí; Romero-Ceronio, Nancy; Roa-de la Fuente, Luis F.; Lobato-García, Carlos E. (2013-10-15). "Synthesis and anti-inflammatory activity of three nitro chalcones". Bioorganic & Medicinal Chemistry Letters. 23 (20): 5519–5522. doi:10.1016/j.bmcl.2013.08.061. ISSN 0960-894X.
- Toda, Fumio; Tanaka, Koichi; Hamai, Koki (1990). "Aldol condensations in the absence of solvent: Acceleration of the reaction and enhancement of the stereoselectivity". Journal of the Chemical Society, Perkin Transactions 1 (11): 3207–9. doi:10.1039/P19900003207.
- Palleros, Daniel R (2004). "Solvent-Free Synthesis of Chalcones". Journal of Chemical Education. 81 (9): 1345. Bibcode:2004JChEd..81.1345P. doi:10.1021/ed081p1345.
- Comisar, Craig M; Savage, Phillip E (2004). "Kinetics of crossed aldol condensations in high-temperature water". Green Chemistry. 6 (4): 227–31. doi:10.1039/b314622g.
- Venkatesan, P; Sumathi, S (2009). "Piperidine mediated synthesis ofn-heterocyclic chalcones and their antibacterial activity". Journal of Heterocyclic Chemistry. 47 (1): 81–84. doi:10.1002/jhet.268.
- Leusink, A.J; Noltes, J.G (1966). "Reaction of organotin hydrides with α,β-unsaturated ketones". Tetrahedron Letters. 7 (20): 2221–5. doi:10.1016/S0040-4039(00)72405-1. hdl:1874/17014.
- Outirite, Moha; Lebrini, Mounim; Lagrenée, Michel; Bentiss, Fouad (2008). "New one step synthesis of 3,5-disubstituted pyrazoles under microwave irradiation and classical heating". Journal of Heterocyclic Chemistry. 45 (2): 503–5. doi:10.1002/jhet.5570450231.
- Zhang, Ze; Tan, Ya-Jun; Wang, Chun-Shan; Wu, Hao-Hao (2014). "One-Pot Synthesis of 3,5-Diphenyl-1H-pyrazoles from Chalcones and Hydrazine under Mechanochemical Ball Milling". Heterocycles. 89: 103–12. doi:10.3987/COM-13-12867.
- Lasri, Jamal; Ismail, Ali I. (2018). "Metal-free and FeCl3-catalyzed synthesis of azines and 3,5-diphenyl-1H-pyrazole from hydrazones and/or ketones monitored by high resolution ESI+-MS". Indian Journal of Chemistry, Section B. 57B (3): 362–373.
- Mahapatra, Debarshi Kar; Bharti, Sanjay Kumar; Asati, Vivek (2017). "Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives". Current Topics in Medicinal Chemistry. 17 (28): 3146–3169. doi:10.2174/1568026617666170914160446. PMID 28914193.
- Xia, Yi; Yang, Zheng-Yu; Xia, Peng; Bastow, Kenneth F.; Nakanishi, Yuka; Lee, Kuo-Hsiung (2000). "Antitumor agents. Part 202: Novel 2′-amino chalcones: design, synthesis and biological evaluation". Bioorganic & Medicinal Chemistry Letters. 10 (8): 699–701. doi:10.1016/S0960-894X(00)00072-X. ISSN 0960-894X. PMID 10782667.
- Santos, Mariana B.; Pinhanelli, Vitor C.; Garcia, Mayara A.R.; Silva, Gabriel; Baek, Seung J.; França, Suzelei C.; Fachin, Ana L.; Marins, Mozart; Regasini, Luis O. (2017). "Antiproliferative and pro-apoptotic activities of 2′- and 4′-aminochalcones against tumor canine cells" (PDF). European Journal of Medicinal Chemistry. 138: 884–889. doi:10.1016/j.ejmech.2017.06.049. hdl:11449/174929. ISSN 0223-5234.
- Mahapatra, D. K., Bharti, S. K., Asati, V., & Singh, S. K. (2017). Perspectives of medicinally privileged chalcone based metal coordination compounds for biomedical applications. European journal of medicinal chemistry, 174, 142-158. DOI: https://doi.org/10.1016/j.ejmech.2019.04.032
- Mahapatra, D. K., Bharti, S. K., & Asati, V. (2015). Anti-cancer chalcones: Structural and molecular target perspectives. European journal of medicinal chemistry, 98, 69-114. DOI: https://doi.org/10.1016/j.ejmech.2015.05.004
- Mahapatra, D. K., Asati, V., & Bharti, S. K. (2015). Chalcones and their therapeutic targets for the management of diabetes: structural and pharmacological perspectives. European journal of medicinal chemistry, 92, 839-865. DOI: https://doi.org/10.1016/j.ejmech.2015.01.051
- Mahapatra, D. K., Bharti, S. K., & Asati, V. (2015). Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. European journal of medicinal chemistry, 101, 496-524. DOI: https://doi.org/10.1016/j.ejmech.2015.06.052
- Mahapatra, D. K., & Bharti, S. K. (2016). Therapeutic potential of chalcones as cardiovascular agents. Life sciences, 148, 154-172. DOI: https://doi.org/10.1016/j.lfs.2016.02.048
- Mahapatra, D. K., Bharti, S. K., & Asati, V. (2017). Chalcone derivatives: Anti-inflammatory potential and molecular targets perspectives. Current topics in medicinal chemistry, 17(28), 3146-3169. DOI: https://doi.org/10.2174/1568026617666170914160446