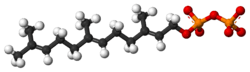
Farnesyl pyrophosphate
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in both the mevalonate and non-mevalonate pathways used by organisms in the biosynthesis of terpenes, terpenoids, and sterols.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(2E,6E)-3,7,11-Trimethyldodeca-2,6,10-triene-1-pyrophosphate | |
| Identifiers | |
| ChemSpider | |
| MeSH | farnesyl+pyrophosphate |
PubChem CID |
|
| UNII | |
| Properties | |
| C15H28O7P2 | |
| Molar mass | 382.326 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is used in the synthesis of CoQ (part of the electron transport chain), as well as being the immediate precursor of squalene (via the enzyme squalene synthase), dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for N-glycosylation), and geranylgeranyl pyrophosphate (GGPP).
Biosynthesis
Farnesyl pyrophosphate synthase (a prenyl transferase)[2] catalyzes sequential condensation reactions of dimethylallyl pyrophosphate with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate, as is shown in the following two steps:
- Dimethylallyl pyrophosphate reacts with 3-isopentenyl pyrophosphate to form geranyl pyrophosphate:
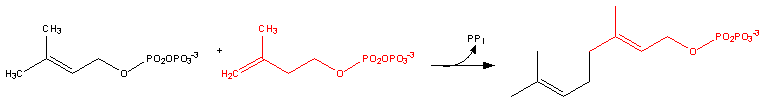
- Geranyl pyrophosphate then reacts with another molecule of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate
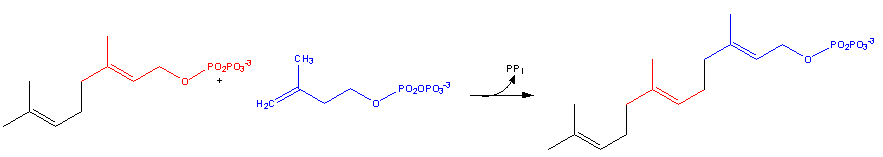
Pharmacology
The above reactions are inhibited by bisphosphonates (used for osteoporosis).[3]
Statin-induced rhabdomyolysis is due to the depletion of farnesyl-PPi, which leads to a depletion of CoQ in the electron transport chain of mitochondria, an organelle that is found in great numbers in myocytes.
Related compounds
References
- Davis, Edward M.; Croteau, Rodney (2000). "Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes". Topics in Current Chemistry. 209: 53–95. doi:10.1007/3-540-48146-X_2. ISBN 978-3-540-66573-1. S2CID 53419212.CS1 maint: uses authors parameter (link)
- Kulkarni RS, Pandit SS, Chidley HG, Nagel R, Schmidt A, Gershenzon J, Pujari KH, Giri AP and Gupta VS, 2013, Characterization of three novel isoprenyl diphosphate synthases from the terpenoid rich mango fruit. Plant Physiology and Biochemistry, 71, 121–131.
- Russell, Graham (April 2006). "Bisphosphonates From Bench to Bedside". Annals of the New York Academy of Sciences. 1068 (April 2006): 367–401. doi:10.1196/annals.1346.041. PMID 16831938. S2CID 20706956.