Pentaerythritol
Pentaerythritol is an organic compound with the formula C(CH2OH)4. Classified as a polyol, it is a white solid. Pentaerythritol is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many other commercial products.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Bis(hydroxymethyl)propane-1,3-diol[1] | |
| Other names
2,2-Bis(hydroxymethyl)1,3-propanediol Pentaerythritol[1] Hercules P 6 Monopentaerythritol Tetramethylolmethane THME PETP Pentaerythrite Pentek Hercules Aqualon improved technical PE-200 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.732 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 |
| Appearance | white solid |
| Density | 1.396g/cm3 |
| Melting point | 260.5 °C (500.9 °F; 533.6 K) |
| Boiling point | 276 °C (529 °F; 549 K) at 30 mmHg |
| 5.6 g/100 mL at 15 °C | |
| Solubility | Soluble in methanol, ethanol, glycerol, ethylene glycol, formamide; insoluble in acetone, benzene, paraffin, ether, CCl4 |
| Vapor pressure | 0.00000008 mmHg (20°C)[2] |
| Hazards | |
| Flash point | 200.1 °C (392.2 °F; 473.2 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
REL (Recommended) |
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
IDLH (Immediate danger) |
N.D.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The word pentaerythritol is a portmanteau of penta- in reference to its 5 carbon atoms and erythritol, which also possesses 4 alcohol groups.
Synthesis
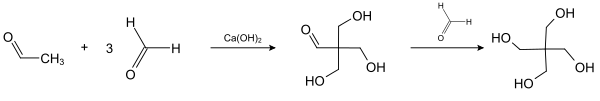
Pentaerythritol was first reported in 1891 by German chemist Bernhard Tollens and his student P. Wigand.[3] It may be prepared via a base-catalyzed multiple-addition reaction between acetaldehyde and 3 equivalents of formaldehyde, followed by a Cannizzaro reaction with a fourth equivalent of formaldehyde to give the final product.[4]
Uses
Pentaerythritol is a versatile building block for the preparation of many polyfunctionalized compounds. Derivatives of pentaerythritol are components of alkyd resins, varnishes, polyvinyl chloride stabilizers, tall oil esters, and olefin antioxidants. It can be found in transformer oil, plastics, paints, cosmetics, and many other applications.[5][6]
Polyester derivatives
Pentaerythritol is a precursor to esters of the type C(CH2OX)4. One such derivative is pentaerythritol tetranitrate (PETN), a vasodilator and explosive. The trinitrate derivative is called pentrinitrol (Petrin). The tetraacetate is called normosterol (PAG). The polymer cross-linking agent pentaerythritol tetraacrylate.[7]
Fire retardants
Pentaerythritol is used as a fire retardant, such as in plastics. It produces a thick carbon barrier upon heating, protecting the surface substrate.
Pentaerythritol is one of the most common main active components in intumescent paints and coatings. It acts as a carbon donor and together with an acid donor, most commonly ammonium polyphosphate (APP), and a blowing agent, most commonly melamine.
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- NIOSH Pocket Guide to Chemical Hazards. "#0485". National Institute for Occupational Safety and Health (NIOSH).
- Tollens, B.; Wigand, P. (1891). "Ueber den Penta-Erythrit, einen aus Formaldehyd und Acetaldehyd synthetisch hergestellten vierwerthigen Alkohol (On pentaerythritol, a quaternary alcohol synthetically produced from formaldehyde and acetaldehyde)". Justus Liebig's Annalen der Chemie (in German). 265 (3): 316–340. doi:10.1002/jlac.18912650303.
- Schurink, H. B. J. (1925). "Pentaerythritol". Organic Syntheses. 4: 53. doi:10.15227/orgsyn.004.0053.; Collective Volume, 1, p. 425
- NPCS Board of Consultants & Engineers (2016). The Complete Book on Adhesives, Glues & Resins Technology (with Process & Formulations) 2nd Revised Edition.
- NIIR Board of Engineers & Consultants (2005). Synthetic Resins Technology Handbook.
- S. F. Marrian (1948). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. PMID 18876970.