Ethylidene diacetate
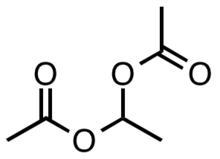
Ethylidene diacetate is an organic compound with the formula (CH3CO2)2CHCH3. A colorless low-melting solid, it once served as a precursor to vinyl acetate.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethane-1,1-diyl diacetate | |
| Other names
1,1-Diacetoxyethane 1,1-Ethanediol diacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.001 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10O4 | |
| Molar mass | 146.14 |
| Appearance | Colorless liquid |
| Density | 1.07 g/cm3 |
| Melting point | 18.9 °C (66.0 °F; 292.0 K) |
| Boiling point | 167–169 °C (333–336 °F; 440–442 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
A major industrial route involves the reaction of acetaldehyde and acetic anhydride in the presence of a ferric chloride catalyst:[1][2]
- CH3CHO + (CH3CO)2O → (CH3CO2)2CHCH3
It can be converted to the valuable monomer vinyl acetate by thermal elimination of acetic acid:
- (CH3CO2)2CHCH3 → CH3CO2CH=CH2 + CH3CO2H
gollark: Go is very satanic, yes.
gollark: Memory-unsafe languages are.
gollark: Yes, exactly, he gets it.
gollark: * apioforms
gollark: r/physicsmemes and just check the first page?
References
- G. Roscher "Vinyl Esters" in Ullmann's Encyclopedia of Chemical Technology, 2007 John Wiley & Sons: New York. doi:10.1002/14356007.a27_419
- GB Patent 238825A 'Process of Manufacture of Acetic Anhydride and Aldehyde'
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.