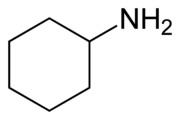
Cyclohexylamine
Cyclohexylamine is an organic compound, belonging to the aliphatic amine class. It is a colorless liquid, although, like many amines, samples are often colored due to contaminants. It has a fishy odor and is miscible with water. Like other amines, it is a weak base, compared to strong bases such as NaOH, but it is a stronger base than its aromatic analog, aniline.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Cyclohexanamine | |
| Other names
Aminocyclohexane Aminohexahydrobenzene Hexahydroaniline Hexahydrobenzenamine | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.300 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H13N | |
| Molar mass | 99.17 |
| Appearance | clear to yellowish liquid |
| Odor | strong, fishy, amine odor |
| Density | 0.8647 g/cm3 |
| Melting point | −17.7 °C (0.1 °F; 255.5 K) |
| Boiling point | 134.5 °C (274.1 °F; 407.6 K) |
| Miscible | |
| Solubility | very soluble in ethanol, oil miscible in ethers, acetone, esters, alcohol, ketones |
| Vapor pressure | 11 mmHg (20° C)[2] |
| Acidity (pKa) | 10.64[3] |
Refractive index (nD) |
1.4565 |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H302, H312, H314, H361 |
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P264, P270, P280, P281, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P308+313, P310, P312, P321, P322, P330 | |
| NFPA 704 (fire diamond) | |
| Flash point | 28.6 °C (83.5 °F; 301.8 K) |
| 293 °C (559 °F; 566 K) | |
| Explosive limits | 1.5–9.4%[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
156 mg/kg (rat, oral) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[2] |
REL (Recommended) |
TWA 10 ppm (40 mg/m3)[2] |
IDLH (Immediate danger) |
N.D.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is a useful intermediate in the production of many other organic compounds. It is a metabolite of cyclamate.
Preparation
Cyclohexylamine is produced by two routes, the main one being hydrogenation of aniline using some cobalt- or nickel-based catalysts:[4]
- C6H5NH2 + 3 H2 → C6H11NH2
It is also prepared by alkylation of ammonia using cyclohexanol.
Applications
Cyclohexylamine is used as an intermediate in synthesis of other organic compounds. It is the precursor to sulfenamide-based reagents used as accelerators for vulcanization. It is a building block for pharmaceuticals (e.g., mucolytics, analgesics, and bronchodilators). The amine itself is an effective corrosion inhibitor. Some sweeteners are derived from this amine, notably cyclamate. The herbicide hexazinone and the anesthetic hexylcaine are derived from cyclohexylamine.[4]
Toxicity
LD50 (rat; p.o.) = 0.71 ml/kg[5]
It is corrosive. Cyclohexylamine is listed as an extremely hazardous substance as defined by Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. It has been used as a flushing aid in the printing ink industry.[6]
The National Institute for Occupational Safety and Health has suggested workers not be exposed to a recommended exposure limit of over 10 ppm (40 mg/m3) over an eight-hour workshift.[2]
References
- Merck Index, 11th Edition, 2735.
- NIOSH Pocket Guide to Chemical Hazards. "#0168". National Institute for Occupational Safety and Health (NIOSH).
- H. K. Hall, J. Am. Chem. Soc. (1957) 79 5441.
- Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- The Merck Index, 10th Ed. (1983) p.392, Rahway: Merck & Co.
- Apps, E. A. (1958). Printing Ink Technology. London: Leonard Hill [Books] Limited. pp. ix.
