Enol
Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.[1]


Biochemistry
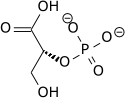
The enzyme enolase catalyzes the dehydration of 2-phosphoglyceric acid to the enol phosphate ester. Metabolism of PEP to pyruvic acid by pyruvate kinase (PK) generates adenosine triphosphate (ATP) via substrate-level phosphorylation.[2]
 |
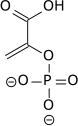 |
 | ||||
| H2O | ADP | ATP | ||||
 |
 | |||||
| H2O | ||||||
Reactivity
The terminus of the double bond in enols is nucleophilic. Its reactions with electrophilic organic compounds underlies the tremendous importance of enol-based intermediates in a wide array of important life processes (i.e., in biochemistry, as intermediates in enzyme-catalysed reactions), as well as being central to modern synthetic organic chemistry (e.g., in applications of aldol and related reactions).
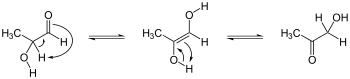
Keto-enol tautomerism
Organic esters, ketones, and aldehydes with an α-hydrogen (C-H bond adjacent to the carbonyl group) often form enols. The reaction involves migration of a proton from carbon to oxygen:[1]
- RC(O)CHR'2 RC(OH)=CR'2
In the case of ketones, the conversion is called a keto-enol tautomerism, although this name is often more generally applied to all such tautomerizations. Usually the equilibrium constant is so small that the enol is undetectable spectroscopically.
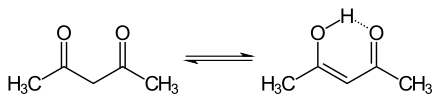
In some compounds with two (or more) carbonyls, the enol form becomes dominant. The behavior of 2,4-pentanedione illustrates this effect:[3]

Enolates
Deprotonation of enolizable ketones, aldehydes, and esters gives enolates.[4][5] With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA).[6] Often, as in conventional Claisen condensations, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low concentrations, but they still undergo reactions with electrophiles. Many factors affect the behavior of enolates, especially the solvent, additives (e.g. diamines), and the countercation (Li+ vs Na+, etc.). For unsymmetrical ketones, methods exist to control the regiochemistry of the deprotonation.[7]
Enolates can also be trapped by acylation and silylation, which occur at oxygen. Silyl enol ethers are common reagents in organic synthesis as illustrated by the Mukaiyama aldol reaction:[8]
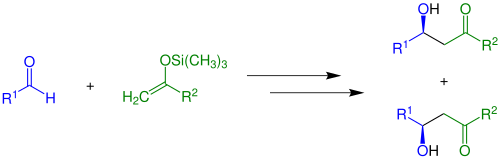
Vinyl acetate is an enol ester that is made on an industrial scale.[9] Lithium enolates adopt aggregated structures akin to other lithium alkoxides.[5]
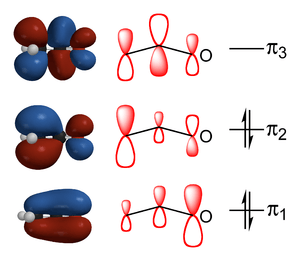
Enolates are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites.[10]
Enediols
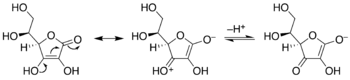
Enediols are alkenes with a hydroxyl group on each carbon of the C=C double bond. Normally such compounds are disfavored components in equilibria with acyloins. One special case is catechol, where the C=C subunit is part of an aromatic ring. In some other cases however, enediols are stabilized by flanking carbonyl groups. These stabilized enediols are called reductones. Such species are important in glycochemistry, e.g., the Lobry de Bruyn-van Ekenstein transformation.[11]
 Keto-enediol tautomerizations. Enediol in the center; acyloin isomers at left and right. Ex. is hydroxyacetone, shown at right.
Keto-enediol tautomerizations. Enediol in the center; acyloin isomers at left and right. Ex. is hydroxyacetone, shown at right.
References
- Smith MB, March J (2001). Advanced Organic Chemistry (5th ed.). New York: Wiley Interscience. pp. 1218–1223. ISBN 0-471-58589-0.
- Berg, Jeremy M.; Tymoczko, Stryer (2002). Biochemistry (5th ed.). New York: W.H. Freeman and Company. ISBN 0-7167-3051-0.
- Manbeck, Kimberly A.; Boaz, Nicholas C.; Bair, Nathaniel C.; Sanders, Allix M. S.; Marsh, Anderson L. (2011). "Substituent Effects on Keto–Enol Equilibria Using NMR Spectroscopy". J. Chem. Educ. 88 (10): 1444–1445. doi:10.1021/ed1010932.
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- Manfred Braun (2015). Modern Enolate Chemistry: From Preparation to Applications in Asymmetric Synthesis. Wiley‐VCH. doi:10.1002/9783527671069. ISBN 9783527671069.
- Christine Wedler, Hans Schick (1998). "Synthesis of Β-lactones By Aldolization of Ketones with Phenyl Ester Enolates: 3,3-Dimethyl-1-oxaspiro[3.5]nonan-2-one". Org. Synth. 75: 116. doi:10.15227/orgsyn.075.0116.CS1 maint: uses authors parameter (link)
- Martin Gall, Herbert O. House (1972). "The Formation and Alkylation of Specific Enolate Anions from an Unsymmetrical Ketone: 2-Benzyl-2-methylcyclohexanone and 2-Benzyl-6-methylcyclohexanone". Org. Synth. 52: 39. doi:10.15227/orgsyn.052.0039.CS1 maint: uses authors parameter (link)
- Mukaiyama, T.; Kobayashi, S. Org. React. 1994, 46, 1. doi:10.1002/0471264180.or046.01
- G. Roscher (2007). "Vinyl Esters". Ullmann's Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_419.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Enolates". doi:10.1351/goldbook.E02123
- Schank, Kurt (1972). "Reductones". Synthesis: 176–90. doi:10.1055/s-1972-21845.