Sodium fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is used in trace amounts in the fluoridation of drinking water, toothpaste, in metallurgy, as a flux, and is also used in pesticides and rat poison. It is a colorless or white solid that is readily soluble in water. It is a common source of fluoride in the production of pharmaceuticals and is used to prevent dental cavities.
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˌsoʊdiəm ˈflʊəraɪd/[1] |
| IUPAC name
Sodium fluoride | |
| Other names
Florocid | |
| Identifiers | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.789 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1690 |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| NaF | |
| Molar mass | 41.988173 g/mol |
| Appearance | White to greenish solid |
| Odor | odorless |
| Density | 2.558 g/cm3 |
| Melting point | 993 °C (1,819 °F; 1,266 K) |
| Boiling point | 1,704 °C (3,099 °F; 1,977 K) |
| 36.4 g/L (0 °C); 40.4 g/L (20 °C); 50.5 g/L (100 °C)[2] | |
| Solubility | slightly soluble in HF, ammonia negligible in alcohol, acetone, SO2, dimethylformamide |
| Vapor pressure | 1 mmHg @ 1077 C°[3] |
| −16.4·10−6 cm3/mol | |
Refractive index (nD) |
1.3252 |
| Structure | |
| Cubic | |
a = 462 pm | |
| Octahedral | |
| Thermochemistry | |
Heat capacity (C) |
46.82 J/mol K |
Std molar entropy (S |
51.3 J/mol K |
Std enthalpy of formation (ΔfH⦵298) |
-573.6 kJ/mol |
Gibbs free energy (ΔfG˚) |
-543.3 kJ/mol |
| Pharmacology | |
| A01AA01 (WHO) A12CD01 (WHO), V09IX06 (WHO) (18F) | |
| Hazards | |
| Safety data sheet | [4] |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H301, H315, H319, H335[4] |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
52–200 mg/kg (oral in rats, mice, rabbits)[5] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 2.5 mg/m3[6] |
REL (Recommended) |
TWA 2.5 mg/m3[6] |
IDLH (Immediate danger) |
250 mg/m3 (as F)[6] |
| Related compounds | |
Other anions |
Sodium chloride Sodium bromide Sodium iodide Sodium astatide |
Other cations |
Lithium fluoride Potassium fluoride Rubidium fluoride Caesium fluoride Francium fluoride |
Related compounds |
TASF reagent |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In 2017, it was the 247th most commonly prescribed medication in the United States, with more than one million prescriptions.[7][8]
Uses

Dental caries
Fluoride salts are often added to municipal drinking water (as well as to certain food products in some countries) for the purpose of maintaining dental health. The fluoride enhances the strength of teeth by the formation of fluorapatite, a naturally occurring component of tooth enamel.[9][10][11] Although sodium fluoride is used to fluoridate water and, indeed, is the standard by which other water-fluoridation compounds are gauged, hexafluorosilicic acid (H2SiF6) and its salt sodium hexafluorosilicate (Na2SiF6) are more commonly used additives in the U.S.[12]
Osteoporosis
Fluoride supplementation has been extensively studied for the treatment of postmenopausal osteoporosis. This supplementation does not appear to be effective; even though sodium fluoride increases bone density, it does not decrease the risk of fractures.[13][14]
Medical imaging
In medical imaging, fluorine-18-labelled sodium fluoride (USP, sodium fluoride F18) is one of the oldest tracers used in positron emission tomography (PET), having been in use since the 1960s.[15] Relative to conventional bone scintigraphy carried out with gamma cameras or SPECT systems, PET offers more sensitivity and spatial resolution. Fluorine-18 has a half-life of 110 min, which requires it to be used promptly once produced; this logistical limitation hampered its adoption in the face of the more convenient technetium-99m-labelled radiopharmaceuticals. However fluorine-18 is generally considered to be a superior radiopharmaceutical for skeletal imaging. In particular it has a high and rapid bone uptake accompanied by very rapid blood clearance, which results in a high bone-to-background ratio in a short time.[16] Additionally the annihilation photons produced by decay of 18F have a high energy of 511-keV compared to 140-keV photons of 99mTc.[17]
Chemistry
Sodium fluoride has a variety of specialty chemical applications in synthesis and extractive metallurgy. It reacts with electrophilic chlorides including acyl chlorides, sulfur chlorides, and phosphorus chloride.[18] Like other fluorides, sodium fluoride finds use in desilylation in organic synthesis. Sodium fluoride can be used to produce fluorocarbons via the Finkelstein reaction; this process has the advantage of being simple to perform on a small scale but is rarely used on an industrial scale due the existence of more effective techniques (e.g. Electrofluorination, Fowler process).
Other uses
Sodium fluoride is used as a cleaning agent (e.g., as a "laundry sour").[19] Sodium fluoride is used as a stomach poison for plant-feeding insects. Inorganic fluorides such as fluorosilicates and sodium fluoride complex magnesium ions as magnesium fluorophosphate. They inhibit enzymes such as enolase that require Mg2+ as a prosthetic group. Thus, fluoride poisoning prevents phosphate transfer in oxidative metabolism.[20]
Safety
The lethal dose for a 70 kg (154 lb) human is estimated at 5–10 g.[19]
Fluorides, particularly aqueous solutions of sodium fluoride, are rapidly and quite extensively absorbed by the human body.[21]
Fluorides interfere with electron transport and calcium metabolism. Calcium is essential for maintaining cardiac membrane potentials and in regulating coagulation. Large ingestion of fluoride salts or hydrofluoric acid may result in fatal arrhythmias due to profound hypocalcemia. Chronic over-absorption can cause hardening of bones, calcification of ligaments, and buildup on teeth. Fluoride can cause irritation or corrosion to eyes, skin, and nasal membranes.[22]
Sodium fluoride is classed as toxic by both inhalation (of dusts or aerosols) and ingestion.[23] In high enough doses, it has been shown to affect the heart and circulatory system. For occupational exposures, the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have established occupational exposure limits at 2.5 mg/m3 over an eight-hour time-weighted average.[24]
In the higher doses used to treat osteoporosis, plain sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause ulcers. Slow-release and enteric-coated versions of sodium fluoride do not have gastric side effects in any significant way, and have milder and less frequent complications in the bones.[25] In the lower doses used for water fluoridation, the only clear adverse effect is dental fluorosis, which can alter the appearance of children's teeth during tooth development; this is mostly mild and is unlikely to represent any real effect on aesthetic appearance or on public health.[26] A chronic fluoride ingestion of 1 ppm of fluoride in drinking water can cause mottling of the teeth (fluorosis) and an exposure of 1.7 ppm will produce mottling in 30–50 % of patients.[21]
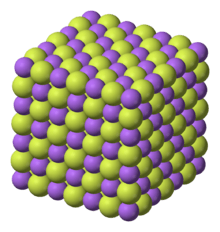
Chemical structure
Sodium fluoride is an inorganic ionic compound, dissolving in water to give separated Na+ and F− ions. Like sodium chloride, it crystallizes in a cubic motif where both Na+ and F− occupy octahedral coordination sites;[27][28] its lattice spacing, approximately 462 pm, is somewhat smaller than that of sodium chloride.
Occurrence
The mineral form of NaF, villiaumite, is moderately rare. It is known from plutonic nepheline syenite rocks.[29]
Production
NaF is prepared by neutralizing hydrofluoric acid or hexafluorosilicic acid (H2SiF6),both byproducts of the reaction of fluorapatite (Ca5(PO4)3F) from phosphate rock during the production of superphosphate fertilizer. Neutralizing agents include sodium hydroxide and sodium carbonate. Alcohols are sometimes used to precipitate the NaF:
- HF + NaOH → NaF + H2O
From solutions containing HF, sodium fluoride precipitates as the bifluoride salt sodium bifluoride (NaHF2). Heating the latter releases HF and gives NaF.
- HF + NaF ⇌ NaHF2
In a 1986 report, the annual worldwide consumption of NaF was estimated to be several million tonnes.[19]
References
- Wells, John C. (2008), Longman Pronunciation Dictionary (3rd ed.), Longman, pp. 313 and 755, ISBN 9781405881180. According to this source, an alternative pronunciation of the second word is /ˈflɔːraɪd/ and, in the UK, also /ˈfluːəraɪd/.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 5.194. ISBN 978-1439855119.
- Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 10th ed. Volumes 1–3 New York, NY: John Wiley & Sons Inc., 1999., p. 3248
- Sigma-Aldrich Co., Sodium Fluoride. Retrieved on 2015-03-17.
- Martel, B.; Cassidy, K. (2004), Chemical Risk Analysis: A Practical Handbook, Butterworth–Heinemann, p. 363, ISBN 978-1-903996-65-2
- NIOSH Pocket Guide to Chemical Hazards. "#0563". National Institute for Occupational Safety and Health (NIOSH).
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Sodium Fluoride - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Bourne, volume editor, Geoffrey H. (1986). Dietary research and guidance in health and disease. Basel: Karger. p. 153. ISBN 978-3-8055-4341-5.
- Jr, Cornelis Klein, Cornelius S. Hurlbut (1999). Manual of mineralogy : (after James D. Dana) (21st ed., rev. ed.). New York: J. Wiley. ISBN 978-0-471-31266-6.
- Selwitz, Robert H; Ismail, Amid I; Pitts, Nigel B (January 2007). "Dental caries". The Lancet. 369 (9555): 51–59. doi:10.1016/S0140-6736(07)60031-2. PMID 17208642.
- Division of Oral Health, National Center for Prevention Services, CDC (1993), Fluoridation census 1992 (PDF), retrieved 2008-12-29.CS1 maint: multiple names: authors list (link)
- Haguenauer, D; Welch, V; Shea, B; Tugwell, P; Wells, G (2000). "Fluoride for treating postmenopausal osteoporosis". The Cochrane Database of Systematic Reviews (4): CD002825. doi:10.1002/14651858.CD002825. PMID 11034769.
- Vestergaard, P; Jorgensen, NR; Schwarz, P; Mosekilde, L (March 2008). "Effects of treatment with fluoride on bone mineral density and fracture risk—a meta-analysis". Osteoporosis International. 19 (3): 257–68. doi:10.1007/s00198-007-0437-6. PMID 17701094.
- Blau, Monte; Ganatra, Ramanik; Bender, Merrill A. (January 1972). "18F-fluoride for bone imaging". Seminars in Nuclear Medicine. 2 (1): 31–37. doi:10.1016/S0001-2998(72)80005-9.
- Ordonez, A. A.; DeMarco, V. P.; Klunk, M. H.; Pokkali, S.; Jain, S.K. (October 2015). "Imaging Chronic Tuberculous Lesions Using Sodium [18F]Fluoride Positron Emission Tomography in Mice". Molecular Imaging and Biology. 17 (5): 609–614. doi:10.1007/s11307-015-0836-6. PMC 4561601. PMID 25750032.
- Grant, F. D.; Fahey, F. H.; Packard, A. B.; Davis, R. T.; Alavi, A.; Treves, S. T. (12 December 2007). "Skeletal PET with 18F-Fluoride: Applying New Technology to an Old Tracer". Journal of Nuclear Medicine. 49 (1): 68–78. doi:10.2967/jnumed.106.037200. PMID 18077529.
- Halpern, D.F. (2001), "Sodium Fluoride", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, doi:10.1002/047084289X.rs071, ISBN 978-0471936237
- Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, Renée; Cuer, Jean Pierre (2005). "Fluorine Compounds, Inorganic". In Ullmann (ed.). Ullmann's Encyclopedia of Industrial Chemistry. Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307. ISBN 978-3527306732.
- Metcalf, Robert L. (2007), "Insect Control", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 9
- Kapp, Robert (2005), "Fluorine", Encyclopedia of Toxicology, 2 (2nd ed.), Elsevier, pp. 343–346
- Greene Shepherd (2005), "Fluoride", Encyclopedia of Toxicology, 2 (2nd ed.), Elsevier, pp. 342–343
- NaF MSDS. hazard.com
- CDC – NIOSH Pocket Guide to Chemical Hazards
- Murray TM, Ste-Marie LG. Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 7. Fluoride therapy for osteoporosis. CMAJ. 1996;155(7):949–54. PMID 8837545.
- National Health and Medical Research Council (Australia). A systematic review of the efficacy and safety of fluoridation [PDF]. 2007. ISBN 1-86496-415-4. Summary: Yeung CA. A systematic review of the efficacy and safety of fluoridation. Evid Based Dent. 2008;9(2):39–43. doi:10.1038/sj.ebd.6400578. PMID 18584000.
- Wells, A.F. (1984), Structural Inorganic Chemistry, Oxford: Clarendon Press, ISBN 978-0-19-855370-0
- "Chemical and physical information", Toxicological profile for fluorides, hydrogen fluoride, and fluorine (PDF), Agency for Toxic Substances and Disease Registry (ATDSR), September 2003, p. 187, retrieved 2008-11-01
- Mineral Handbook (PDF), Mineral Data Publishing, 2005.
