Zinc acetate
Zinc acetate is a salt with the formula Zn(CH3CO2)2, which commonly occurs as the dihydrate Zn(CH3CO2)2·2H2O. Both the hydrate and the anhydrous forms are colorless solids that are commonly used in chemical synthesis and as dietary supplements. Zinc acetates are prepared by the action of acetic acid on zinc carbonate or zinc metal. When used as a food additive, it has the E number E650.
| Names | |
|---|---|
| IUPAC name
Zinc acetate | |
| Other names
Acetic acid, Zinc salt Acetic acid, Zinc(II) salt Dicarbomethoxyzinc Zinc diacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.338 |
| E number | E650 (flavour enhancer) |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Zn(CH3COO)2(H2O)2 (dihydrate) | |
| Molar mass | 219.50 g/mol (dihydrate) 183.48 g/mol (anhydrous) |
| Appearance | White solid (all forms) |
| Density | 1.735 g/cm3 (dihydrate) |
| Melting point | Decomposes at 237 °C (459 °F; 510 K) (dihydrate loses water at 100 °C) |
| Boiling point | decomposes |
| 43 g/100 mL (20 °C, dihydrate) | |
| Solubility | 1.5 g/100 mL (methanol) |
| −101.0·10−6 cm3/mol (+2 H2O) | |
| Structure | |
| octahedral (dihydrate) | |
| tetrahedral | |
| Pharmacology | |
| A16AX05 (WHO) | |
| Hazards | |
| Main hazards | mildly toxic |
| R-phrases (outdated) | R22 R36 R50/53 |
| S-phrases (outdated) | S26 S60 S61 |
| Related compounds | |
Other anions |
Zinc chloride |
Other cations |
Copper(II) acetate |
Related compounds |
Basic beryllium acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Dietary and medicinal applications
Zinc acetate has been used in lozenges for treating the common cold.[1] Zinc acetate can also be used to treat zinc deficiencies.[2] As an oral daily supplement it is used to inhibit the body's absorption of copper as part of the treatment for Wilson's disease.[3] Zinc acetate is also sold as an astringent in the form of an ointment, a topical lotion, or combined with an antibiotic such as erythromycin for the topical treatment of acne.[4] It is commonly sold as a topical anti-itch ointment.
Industrial applications
Industrial applications include wood preservation, manufacturing other zinc salts, polymers, manufacture of ethyl acetate, as a dye mordant, and analytical reagent. It is used in commercial nuclear power plants as a plating inhibitor on primary water piping.
Basic properties and structures
In anhydrous zinc acetate the zinc is coordinated to four oxygen atoms to give a tetrahedral environment, these tetrahedral polyhedra are then interconnected by acetate ligands to give a range of polymeric structures.[5][6][7] In contrast, most metal diacetates feature metals in octahedral coordination with bidentate acetate groups.
In zinc acetate dihydrate the zinc is octahedral, wherein both acetate groups are bidentate.[8][9]
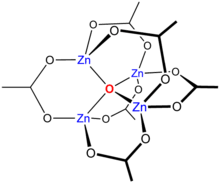
Basic zinc acetate
Heating Zn(CH3CO2)2 in a vacuum results in a loss of acetic anhydride, leaving a residue of basic zinc acetate, with the formula Zn4O(CH3CO2)6. This cluster compound has the tetrahedral structure shown below. This species closely resembles the corresponding beryllium compound, although it is slightly expanded with Zn-O distances ~1.97 vs ~1.63 Å for Be4O(OAc)6.[10]
See also
- Basic beryllium acetate - isostructural
References
- "Zinc – Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. February 11, 2016. Retrieved November 15, 2018.
- Wegmüller, Rita; Tay, Fabian; Zeder, Christophe; Brnić, Marica; Hurrell, Richard F. (2014). "Zinc Absorption by Young Adults from Supplemental Zinc Citrate is Comparable with That from Zinc Gluconate and Higher than from Zinc Oxide". The Journal of Nutrition. 144 (2): 132–136. doi:10.3945/jn.113.181487. PMC 3901420. PMID 24259556.
- "Wilson Disease". NIDDK. July 2014. Archived from the original on 2016-10-04. Retrieved November 15, 2018.
- Schachner, L.; Eaglstein, W.; Kittles, C.; Mertz, P. (1990). "Topical erythromycin and zinc therapy for acne". Journal of the American Academy of Dermatology. 22 (2 Pt 1): 253–60. PMID 2138176.
- Clegg, W.; Little, I. R.; Straughan, B. P. (15 December 1986). "Monoclinic anhydrous zinc(II) acetate". Acta Crystallographica Section C. 42 (12): 1701–1703. doi:10.1107/S010827018609087X.
- He, Hongshan (15 November 2006). "A new monoclinic polymorph of anhydrous zinc acetate". Acta Crystallographica Section E. 62 (12): m3291–m3292. doi:10.1107/S1600536806046678.
- Capilla, A. V.; Aranda, R. A. (1979). "Anhydrous Zinc(II) Acetate (CH3-COO)2Zn". Crystal Structure Communications. 8: 795–797.
- van Niekerk, J. N.; Schoening, F. R. L.; Talbot, J. H. (10 September 1953). "The crystal structure of zinc acetate dihydrate, Zn(CH3COO)2.2H2O". Acta Crystallographica. 6 (8): 720–723. doi:10.1107/S0365110X53002015.
- Ishioka, T.; Murata, A.; Kitagawa, Y.; Nakamura, K. T. (15 August 1997). "Zinc(II) Acetate Dihydrate". Acta Crystallographica Section C. 53 (8): 1029–1031. doi:10.1107/S0108270197004484.
- Koyama, H.; Saito, Y. (1954). "The Crystal Structure of Zinc Oxyacetate, Zn4O(CH3COO)6". Bull. Chem. Soc. Jpn. 27 (2): 112–114. doi:10.1246/bcsj.27.112.